Article Text
Abstract
Background Asthma guidelines recommend monitoring of asthma control. However, in a substantial proportion of children, asthma is poorly controlled and the best monitoring strategy is not known.
Objectives We studied two monitoring strategies for their ability to improve asthma outcomes in comparison with standard care (SC): web-based monthly monitoring with the (Childhood) Asthma Control Test (ACT or C-ACT) and 4-monthly monitoring of FENO.
Methods In this randomised controlled, partly blinded, parallel group multicentre trial with a 1-year follow-up, children aged 4–18 years with a doctor's diagnosis of asthma treated in seven hospitals were randomised to one of the three groups. In the web group, treatment was adapted according to ACT obtained via a website at 1-month intervals; in the FENO group according to ACT and FENO, and in the SC group according to the ACT at 4-monthly visits. The primary endpoint was the change from baseline in the proportion of symptom-free days (SFD).
Results Two-hundred and eighty children (mean age 10.4 years, 66% boys) were included; 268 completed the study. Mean changes from baseline in SFD were similar between the groups: −2.1% (web group, n=90), +8.9% (FENO group, n=91) versus 0.15% (SC, n=87), p=0.15 and p=0.78. Daily dose of inhaled corticosteroids (ICS) decreased more in the web-based group compared with both other groups (−200 μg/day, p<0.01), while ACT and SFD remained similar.
Conclusions The change from baseline in SFD did not differ between monitoring strategies. With web-based ACT monitoring, ICS could be reduced substantially while control was maintained.
Trial registration number NTR 1995.
- Paediatric asthma
Statistics from Altmetric.com
Key messages
What is the key question?
-
Does web-based monthly monitoring with the (Childhood) Asthma Control Test or 4-monthly monitoring of FENO improve asthma outcomes in comparison with standard care?
What is the bottom line?
-
This randomised controlled trial shows that neither monthly web-based monitoring with the Asthma Control Test nor regular measurement of FENO increased symptom-free days in asthmatic children.
Why read on?
-
Despite the emphasis of asthma guidelines on monitoring childhood asthma, the best way of how to do this is still unknown; this study evaluates three monitoring strategies.
Introduction
In a substantial proportion of children with asthma, control is not achieved.1–3 Guidelines pay little attention to monitoring strategies. The Asthma Control Test (ACT) and the Childhood ACT (C-ACT) for children aged <12 years are simple, validated tools to assess asthma control.4 ,5 Web-based ACT monitoring of asthma control has the potential to standardise the assessment of asthma control, increase awareness of asthma and improve adherence. The fraction of nitric oxide in exhaled air (FENO) is a non-invasive biomarker of eosinophilic airway inflammation6 but the benefits of FENO for asthma monitoring are not yet clear.7 In the present study, we evaluated the possible benefits of web-based monitoring with monthly ACT measurements and adapting the treatment based on 4-monthly FENO measurements. We hypothesised that both strategies are superior to standard care (SC).
Methods
The ‘Better Asthma Treatment: Monitoring with ACT and Nitric oxide’ study was a multicentre, prospective, partly blinded, parallel-group, three-arm randomised controlled superiority trial on monitoring strategies in asthmatic children with a follow-up of 1 year (trial number NTR 1995).
Patients
Children aged 4–18 years, with atopic asthma based on clinical symptoms, a previous bronchodilator response of >9% increase in FEV1 of predicted (FEV1%) and/or previous airway hyper-responsiveness (AHR) to methacholine were recruited by their own paediatrician from general hospitals (n=5) and tertiary referral centres (n=2) in the Netherlands from February 2010 to November 2011. Atopy was defined as a radioallergosorbent test class 2 or higher for at least one airborne allergen. Patients had been using inhaled corticosteroids (ICS) for at least 3 months before the study. Exclusion criteria were active smoking, pulmonary diseases other than asthma, recent (<1 year) or multiple admissions to an intensive care unit for asthma, inability to perform FENO measurements and/or the use of omalizumab. All parents and children aged ≥12 years gave written informed consent.
Study design
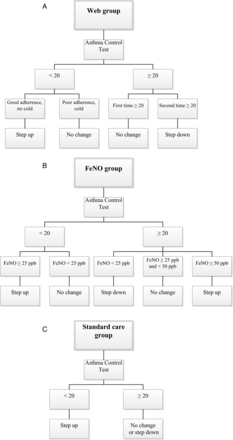
Patients were screened for eligibility by the physician and enrolled by the local investigator, who collected data on subject characteristics, FENO and ACT score (visit 0). After a 4-week run-in period, children were automatically and randomly allocated to one of the three groups by a randomisation programme on the study website, in a 1:1:1 ratio, stratified for age (<12 or ≥12 years), centre and dose of ICS (<400 or ≥400 µg budesonide or equivalent daily dose; figure 1). Children who completed <50% of the diaries in the run-in period were withdrawn from the study. In the web group, treatment was adapted monthly according to the web-based ACT score, while in the FENO group, treatment was adapted to FENO and ACT score at clinic visits every 4 months (figure 2). In the FENO group, we used two cut-off points for decreasing (<25 ppb) or increasing (>50 ppb) treatment. In the SC group, the ACT score during clinic visits directed treatment (figure 2). In case of ACT ≥20 step down was advised, but the physician was allowed to leave treatment unchanged. The study duration was 12 months, with visits at 4-month intervals and one baseline visit 4 weeks prior to randomisation. In addition, children randomised to the web group were asked to fill in an ACT every month on a website that provided a treatment advice by email by the researcher or asthma nurse within three working days. In the FENO and SC groups, treatment changes were possible at the 4-monthly study visits; in the web group, treatment could be changed monthly. Patients were not blinded to randomisation group. The treating physicians were blinded to randomisation group, FENO and ACT. The local investigators, unblinded to ACT and FENO, provided the physicians with treatment advice based on the study algorithms and on the treatment plan (figure 2; see online supplementary table S1). Physicians could deviate from this advice for documented clinical reasons only. The primary endpoint was change from baseline of the proportion of symptom-free days (SFD); secondary endpoints were changes from baseline of ACT, daily symptom score, daily bronchodilator doses, daily ICS dose, asthma-related quality of life, dose of methacholine causing a 20% fall in FEV1 (PD20), FVC, FEV1 and maximal expiratory flow at 25% of vital capacity (MEF25) and exacerbations during the study. The final ICS dose was the dose the patient was on immediately prior to the final visit.
Study design. At visit 1, children were randomly allocated to one of the three study groups.
Study algorithm of (A) the web group, (B) the FENO group and (C) the standard care group. In the web group, patients were asked about adherence to treatment and on signs of airways infection if they returned an Asthma Control Test (ACT) or Childhood ACT (C-ACT) <20. If adherence was poor, or patients had clinical signs of an airway infection (runny nose, fever and malaise), there was no step-up in treatment.
Symptom-free days
SFDs were defined as days without daytime or night-time symptoms and were obtained from a web-based diary which recorded daytime and night-time symptoms, limitation in activity and use of reliever medication.8 Diary data were collected twice daily during the 4-week run-in period, during the 2 weeks preceding each visit and during 4 weeks preceding the final visit (figure 1). Each symptom item had four response levels (0=asymptomatic to 3=highly symptomatic); the maximal daily diary score was 21. Diaries were automatically date-stamped and time-stamped and could be filled in until 5 days after the date. Participants received a reminder by email once daily.
Asthma Control Test
We used the Dutch, translated and linguistically validated version of the ACT (MAPI-research institute, Lyon, France) in children from the age of 12 years, and the C-ACT for children aged 4–11 years.4 ,5
Lung function and FENO
FVC, FEV1 and MEF25 were assessed using an electronic spirometer (Masterscreen, Jaeger, Würzburg, Germany) and expressed as percentage predicted or z-score according to GLI2012.9 The provocative dose of methacholine that produced a 20% fall in FEV1 (PD20) was measured using the dosimeter method (see details in the online supplement). FENO was measured online on the NIOX chemiluminescence analyzer or NIOX MINO (Aerocrine, Stockholm, Sweden) according to guidelines.10
Quality of life
In children aged 12 years and older, asthma-related quality of life was measured with the 23-item self-reported Dutch validated version of the Paediatric Asthma-Related Quality of Life Questionnaire (PAQLQ) for children11 ,12 and expressed as overall asthma-related quality of life. In children aged below 12 years, we used the Paediatric Asthma-Related Caregiver Quality of Life Questionnaire (PACQLQ).11 In both tests, the maximal score of 7 indicates optimal quality of life.
Statistical analysis
Because there were two major comparisons in this study (Web vs SC and FENO vs SC), the alpha level was set at two-sided 0.025. For all other comparisons, the limit of significance was set at two-sided p=0.05.The power of the study was calculated to detect differences between the experimental arms and SC, based on 18% increase in the mean percentage of SFD (corresponding to 2.5 extra SFD in 2 weeks time) with 80% probability in 87 children per group.13 Baseline data were compared using the χ2 test for categorical data or the Kruskal–Wallis test for continuous data. Diary data were evaluated using repeated measurements analysis of variance with baseline data during run-in used as covariates. The significance of differences of other continuous endpoints was determined using analysis of covariance (adjusted for baseline). For within-group comparisons, we used paired t tests. The statistical analysis of PD20 is described in the online supplement. Data were analysed with SPSS for Windows, V.21.0.
Results
Of the 481 children who were eligible for participation, 201 refused, mostly because they were too busy with school (figure 3). Eight children could not be randomised because of non-adherence. Thus, 272 were included: 91 in the web group, 92 in the FENO group and 89 in the SC group. Baseline characteristics are given in table 1. The drop-out rate during the trial was small in both groups (web: n=1, FENO: n=1; SC: n=2), all related to non-compliance. The proportions of children with well-controlled asthma (ACT≥20) at the start of the study were 0.77 for the web group, 0.73 for the FENO group and 0.76 for the SC group (NS). Baseline ACT was significantly higher in the web group compared with the FENO group, but in both intervention groups, ACT was not significantly different from the SC group.
Patient characteristics
Patient flow according to CONSORT guidelines. A total of 481 children were approached to participate in the study, of which 201 refused. A total of eight children could not be randomised. In the web group, one dropped out; in the FENO group, one dropped out; in the standard care group, two children dropped out.
Symptom-free days
The change in the proportion of SFD between visits 1 and 4 did not differ significantly between the groups (SC vs web group: 0.07 (p=0.2); SC vs FENO: 0.01 (p=0.8)). A small but significant within-group change was seen both in the FENO group and in the SC group: 0.53 to 0.62 (p=0.01) and 0.54 to 0.61 (p=0.04), respectively (table 2). Longitudinal data are presented in the online supplement.
Changes from baseline of different outcome parameters
ACT scores
The FENO group showed a 1.7-point improvement in ACT that was higher than in the SC group (0.4 points) (p=0.02). This change in (C-) ACT points was determined by the findings in the young children who used the C-ACT: 1.84 points (p<0.01), while no such change was present in children who used the ACT (−0.28 points, p=0.7). No significant change in the ACT was seen in the web group.
Symptom scores, limitation of activities and use of β-2 agonists
The change between visits 1 and 4 in mean daily symptom scores and the use of β-2 agonists did not differ between the SC and the web group or between the SC and the FENO group (table 2). The mean daily symptom score improved within the FENO group only (−0.40, p=0.01).
Exacerbations
There were 42 exacerbations during the study: 7 hospital admissions and 35 systemic steroid courses. The distribution over the groups was similar: 10, 14 and 17 exacerbations in the web, FENO and SC groups, respectively. The exacerbation number per patient per year was 0.11 (web group), 0.15 (FENO group), 0.20 (SC); differences were not significant.
Lung function and FENO
Mean changes from baseline in lung function and PD20 did not differ between groups (table 2). FEV1 and FVC increased significantly in both the FENO and the SC group. Data on PD20 are presented in the online supplement. Within all groups, a significant increase in FENO was found. The geometric means of FENO increased by a factor of 1.6 in the web group, 1.4 in the FENO group (both p<0.01) and 1.2 in the SC group (p=0.04). Changes in FENO during the study were significantly different between the SC and the web group, but not between the SC and the FENO group. The ratios of geometric means, adjusted for baseline, were 1.3 (p<0.01) for the web group and 1.1 for the FENO group (p=0.24) as compared with the SC group.
Treatment levels and ICS doses
At visit 1, median ICS doses, long-acting β-agonist use and leukotriene receptor antagonist use did not differ between the study groups (table 1). In all groups, medication could be reduced. Medication could be reduced by 74% in children in the web group, 52% in the FENO group and 45% in the SC group. The reduction was significantly larger in the web group compared with the SC group (p<0.001), but not in the FENO group. The mean daily ICS dose was reduced by 201 μg in the web group, 107 μg in the FENO group and 54 μg in the SC group (both p<0.01, table 2). Longitudinal data are presented in the online supplement.
Quality of life
In all groups, there were no significant changes in PAQLQ or PACQLQ scores, and mean changes from baseline did not differ between the groups (table 2).
Decision making
For the FENO group and the SC group, we determined whether the treatment advice would have been different if patients had been allocated to the other study group, which was the case in 62% of all decisions.
Adherence of patients and physicians
The completion rates of the web-based diary cards, web-based ACTs, medication adherence and physicians’ adherence to the protocol were excellent (see online supplement).
In online supplementary table S2, absolute values of secondary outcomes at the start and end of the study are presented.
Discussion
We compared web-based ACT monitoring and FENO monitoring with SC in children with allergic asthma. After 1 year, both strategies had not improved the number of SFD more than SC. Monthly web-based ACTs resulted in a clinically relevant decrease of ICS dose, while maintaining asthma control. Children in the FENO group showed a modest increase in (C-)ACT score as compared with SC, with similar average medication levels. This increase in asthma control was only found in the children under the age of 12 years who used the C-ACT. Other measures of asthma control did not improve as a result of FENO monitoring.
The ACT is increasingly used in asthma management and the Global Initiative for Asthma guidelines state that ‘the value of ACT and C-ACT in clinical use has yet to be demonstrated but will likely become evident in coming years.’2 This study does not support the clinical use of monthly ACT measurements as our primary outcome did not improve. However, we were able to decrease the dose of ICS significantly only in the web group, and this suggests that ACT-guided step-down of treatment is feasible and safe, and reduces overtreatment. By administering the ACT once monthly, a fast response to changes in asthma control is possible. The higher frequency of healthcare contacts may explain part of the success of this approach. Web-based monitoring is a promising new strategy in the care for patients with a chronic illness, including asthma. It enables for remote delivery of care, facilitating timely access to healthcare, supporting self-monitoring, medication adherence and education.14–18 Earlier studies showed improved lung function, increased adherence, improved asthma control, increased knowledge and improved quality of life after web-based monitoring of asthma compared with SC.19–21 We did not find improved asthma control with web-based ACT monitoring. One might argue that baseline ACT, PAQLQ and lung function were higher in the web group than in the SC group, suggesting that the children in the web group had better controlled asthma. Hence, they may have had a greater likelihood of successful ICS reduction. However, the difference between ACT at baseline and the change in ACT from baseline was not significantly different for both groups. This simple strategy halved the dose of ICS, which is substantial, potentially cost-saving and may prevent overtreatment and limit side effects like reduced growth velocity. We did not observe effects on growth: changes in height between the web group and SC group were not significant (data not shown). During the study, FENO increased in the web group when compared with the SC group to a median of 25.2 ppb, which is a common value in well-controlled asthma. This might be explained by the decrease in ICS and by regression to the mean.
A meta-analysis on 21 randomised controlled trials on telemonitoring interventions in both children and adults with asthma concluded that telemonitoring was comparable with SC regarding quality of life, emergency room visits and hospitalisations for asthma.22 A recent review on digital asthma self-management interventions concluded that these interventions are promising, with evidence of beneficial effects.23 Differences in populations, web-based interventions or frequency of internet contact may account for these discrepancies. The relatively low monitoring frequency in our study might have contributed to the high compliance rates and positive effect. The application of web-based monitoring in childhood asthma management has previously been shown feasible and was well accepted.20 ,22 ,24 ,25 In our study, compliance with web-based ACT monitoring was high and patients were generally satisfied with the web-based strategy, in line with earlier studies.24 ,25 Future research should focus on determining which patients will benefit most from web-based interventions, to achieve personalised management.
Several studies addressed titration of ICS treatment on FENO as a marker of eosinophilic inflammation.13 ,26–28 Our primary endpoint, SFD and other markers of asthma control did not improve more with FENO monitoring than with SC. We did observe that FENO monitoring improved the C-ACT scores in children aged from 4 to 11 years with similar doses of ICS as compared with SC. The ACT increase of 1.8 points in this age group is modest but clinically relevant.8 A recent systematic review of four paediatric studies showed no differences in exacerbations, symptoms or lung function between FENO-based and symptom-based monitoring.6 ,7 ,26–28 In a recent study, FENO-guided asthma management did not improve the proportion of SFD, which is in line with our study, but did result in fewer exacerbations.29 How does the current study differ from earlier studies? First, we used two FENO cut-off points to define treatment adjustments, in contrast to other FENO dose titration studies, but similar to the study of Szefler et al.26 The use of two cut-offs to decrease (<25 ppb) or increase (>50 ppb) the dose of ICS is more in accordance with clinical practice. While FENO >25 ppb may be abnormal in healthy subjects, in patients with well-controlled asthma, such a value is common, and a growing body of evidence suggests that cut-offs should be based on FENO levels characteristic of the population of interest.30 In our study population, children with well-controlled asthma had a median FENO level of 25 ppb (IQR 16–47). Therefore, a cut-off of 25 ppb might not be optimal to increase the dose of ICS in asthmatic children. Several studies have shown that 50 ppb seems more appropriate.31 Second, in our study, we could decrease ICS when FENO values were low, while most earlier studies did not allow for this. Third, and probably most important, the treatment algorithms based on FENO and SC differed substantially: taking FENO into account led to a different treatment decision at five of the eight possible treatment changes (discordance–concordance ratio 1.7).32 In our study, including FENO in the treatment algorithm had actually influenced 62% of all treatment decisions.
Unexpectedly, only the younger children appeared to have benefit from the FENO strategy. One might speculate that overestimation of asthma control by the parents may play a role and that FENO helps to assess asthma control more objectively.33
Are our findings generalisable to general paediatric asthma care? Almost half of all eligible children refused to participate in the study, which may limit the conclusions due to selection bias. However, these children did not differ from included children regarding baseline characteristics. A limitation might be bias due to the required internet access. However, more than 95% of Dutch households have internet access at home, and only a single eligible child was excluded for this reason.
In conclusion, we have shown that web-based monitoring with monthly ACT or 4-monthly FENO in children with asthma was feasible but did not improve SFD, our primary outcome. Web-based monitoring led to a significant and clinically relevant reduction in ICS dose with similar asthma control, suggesting better, personalised asthma management. FENO monitoring modestly improved asthma control selectively in children aged <12 years without the need for higher ICS doses. We hypothesise that a combined strategy of web visits in combination with FENO measurements during clinic visits may be a superior strategy to monitor children with asthma, and this should be tested in future studies.
Acknowledgments
We gratefully thank the involved paediatricians, paediatric asthma nurses and lung function technicians for their help and active guidance throughout the study: Simone Suelmann, Hannie Achterberg, Bas Harzing, Dorine de Hond, Saskia ten Raa (Amphia Hospital), Coosje Sintnicolaas, Cindy Hugen, Jessie Jacobs, Petra Theissen (Radboud University Medical Center), Christel Linssen, Martin Claassens (Catharina Hospital), Linda van Gilst, Gracé Theunissen, Mark Holewijn, Annejet Plaisier (Rijnstate Hospital), Renata de Ridder, Else Stoter (De Kinderkliniek), Prof. Wim van Aalderen, Saeeda Lone and Erik-Jonas van de Griendt (Amsterdam Medical Center).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
-
Contributors SV-vB, JCdJ and MWP: Operated as a writing committee and drafted the manuscript until it reached its final form. SV-vB, AAV-V, HJB, AML, NJvdB, PJM, MWP and JCdJ: All participated as investigators in this trial, recruited patients, performed the treatments and commented on the manuscript and its revisions. SV-vB and WCH: Performed the statistical analysis.
-
Funding This study was funded by Lung foundation Netherlands (grant no 3.4.08.039), the Netherlands Organization for Health Research (ZonMW) (grant no 171002101), and Fund Nuts Ohra (grant no 0901-023).
-
Competing interests None declared.
-
Ethics approval Medical Ethics Committee of the Erasmus University Medical Centre, Rotterdam.
-
Provenance and peer review Not commissioned; internally peer reviewed.