Article Text
Abstract
With the improving survival of patients with cystic fibrosis (CF), the clinical spectrum of this complex multisystem disease continues to evolve. One of the most important clinical events for patients with CF in the course of this disease is an acute pulmonary exacerbation. Clinical and microbial epidemiology studies of CF pulmonary exacerbations continue to provide important insight into the course, prognosis and complications of the disease. This review provides a summary of the pathophysiology, clinical epidemiology and microbial epidemiology of a CF pulmonary exacerbation.
- ARIC, Acute Respiratory Illness Checklist
- CF, cystic fibrosis
- CFTR, cystic fibrosis transmembrane regulator
- FEV1, forced expiratory volume in 1 s
- IL, interleukin
- MCBT, multiple combination bactericidal testing
- RSSQ, Respiratory and Systemic Symptoms Questionnaire
- TNFα, tumour necrosis factor α
Statistics from Altmetric.com
- ARIC, Acute Respiratory Illness Checklist
- CF, cystic fibrosis
- CFTR, cystic fibrosis transmembrane regulator
- FEV1, forced expiratory volume in 1 s
- IL, interleukin
- MCBT, multiple combination bactericidal testing
- RSSQ, Respiratory and Systemic Symptoms Questionnaire
- TNFα, tumour necrosis factor α
With the improving survival of patients with cystic fibrosis (CF), the clinical spectrum of this complex multisystem disease continues to evolve. One of the most important clinical events for patients with CF in the course of this disease is an acute pulmonary exacerbation. The primary goal of this review is to provide a summary of the pathophysiology, clinical epidemiology and microbial epidemiology of a CF pulmonary exacerbation. Previous reviews of this subject have been done, but without the focus on both clinical and microbial epidemiology.1
Pathophysiology of CF pulmonary exacerbation
Much of the morbidity and mortality associated with CF is related to the pulmonary system, primarily the upper and lower airways. Extensive basic research has advanced our understanding of the primary defect in CF (mutations in the cystic fibrosis transmembrane regulator (CFTR)) and the potential pathophysiological implications of this defect. Although no definitive work has fully explained how mutations in CFTR lead to airways disease in CF, the most prominent hypothesis to explain this phenomenon is the “low volume hypothesis”.2,3 Based on in vitro data, the proposed mechanism of CF airways disease is that, because of sodium hyperabsorption and lack of chloride absorption, the volume of the periciliary lining fluid decreases with relative dehydration of the layer. This leads to low volume of fluid, impaired cilary function and slower mucociliary transport allowing bacterial overgrowth. With infection, neutrophils are recruited to the airway with subsequent release of pro-inflammatory cytokines leading to a vicious cycle of chronic infection and inflammation that ultimately injures the airways. The supportive evidence for this in vitro hypothesis comes from a mouse model of CF lung disease in which epithelial sodium channels are overexpressed4 and clinical trials in patients with CF.5,6 A more recent additional model that may be compatible with this “low volume hypothesis” relates to the evidence suggesting a lack of mucus secretion, potentially due to defective anion-mediated fluid absorption within the CF airway glands.7–,9
Despite our detailed understanding of CF at a cellular level, very little is known about the pathophysiology of recurrent episodes of increasing pulmonary symptoms termed exacerbations. Exacerbations of pulmonary disease are very common and present clinically with changes in cough, sputum production, dyspnoea, decreased energy level and appetite, weight loss and decreases in spirometric parameters. These episodes are probably related to a complex relationship between host defence and airway microbiology that impacts on sputum production and airflow obstruction. Viral infections, including respiratory syncytial virus, may play a role in the initiation of these events,10 although data regarding the impact of vaccination against viral infection are limited.11,12 Pulmonary exacerbations have also been associated with the acquisition of new organisms or with a change in the bacterial density of colonising flora.13–,16 Bacterial concentrations of Pseudomonas aeruginosa are high during an exacerbation and decrease with treatment; and treatment with antimicrobial agents reduces symptoms and improves lung function.13,14,17 Interestingly, recent data suggest that the majority of exacerbations are not due to acquisition of new strains of Pseudomonas but a clonal expansion of existing strains.18 An inflammatory response in the airway in conjunction with the increase in bacterial concentration and polymorphonucleocytes has been documented with increases in interleukin (IL)8, IL6, IL1β, tumour necrosis factor (TNF)α, leukotriene B4 and free neutrophil elastase; these inflammatory mediators have been noted to decrease with treatment of the pulmonary exacerbation.19–,24 Recent controlled clinical trials have shown that mucolytics, inhaled aminoglycosides, oral macrolides and inhaled hypertonic saline all reduce the rate of pulmonary exacerbations in CF.17,25–,27
Clinical epidemiology of CF pulmonary exacerbation
Definitions/diagnosis
Despite calls for a consensus diagnosis of pulmonary exacerbations by a CF Outcomes Group in 1994 and in a more recent editorial,28,29 no consensus diagnostic criteria exist. Clinical diagnostic criteria commonly used can be found in the Cystic Fibrosis Foundation clinical practice guidelines.30 Definitions have been used in major clinical trials evaluating new treatments in CF. These definitions have been based on empirical data, but have not been formally validated (fig 1⇓).17,25,27 They have combined patient symptomotology, laboratory data and clinician evaluation.17,25 These definitions of a pulmonary exacerbation have revolved around the clinician’s decision to treat a constellation of symptoms, but a treatment decision-defined outcome is, by its nature, problematic. Within the US, practice patterns are far from uniform with regard to the treatment decision for a pulmonary exacerbation,31 so using a treatment decision in the definition is a problem.
Two additional scoring systems were developed to diagnose pulmonary exacerbations and were used in two recent phase 2 CF clinical trials. The first score used was the Acute Respiratory Illness Checklist (ARIC).11,12 It was used as a symptom score to identify patients with lower respiratory tract infections, with the goal of capturing a wider spectrum of CF exacerbations in study participants. The second diagnostic score was the Respiratory and Systemic Symptoms Questionnaire (RSSQ; M W Konstan, personal communication); this score was created to have a uniform approach to identifying CF-related pulmonary exacerbations including mild events not necessitating intravenous antibiotics. Table 1⇓ compares the signs and symptoms assessed in these four different instruments to diagnose a CF pulmonary exacerbation. Despite this work, no consensus has been reached regarding the diagnostic criteria for a pulmonary exacerbation.
Symptom profiles used in various definitions of a pulmonary exacerbation
Components of these definitions have been examined to see which clinical characteristics best predict a pulmonary exacerbation.32–,34 Rosenfeld and colleagues, using data from a clinical trial, used a multivariate modelling approach to create an algorithm to identify participants with a pulmonary exacerbation.33 Symptoms rather than physical examination and laboratory values were found to be more predictive of a pulmonary exacerbation. Two additional studies have noted very similar results.32,34 The signs and symptoms that were most predictive of a pulmonary exacerbation in all of these studies were increased cough, change in sputum (volume or consistency), decreased appetite or decreased weight, change in respiratory examination and respiratory rate.1 This work points to the clear need to focus on signs and symptoms in any future consensus diagnostic criteria.
Mild versus severe exacerbations
Mild CF pulmonary exacerbations have received very little attention and no clear definition exists in the literature. There is a spectrum of clinical presentations of exacerbations from clinical events managed as an outpatient to those requiring admission to an intensive care unit (ICU). One could hypothesise that the mild CF pulmonary exacerbation might present as an early precursor of a severe exacerbation, a milder version of a severe exacerbation or an isolated clinical event that does not evolve into a clear lower respiratory tract infection. Detailed natural history data may elucidate which of these patterns predominates and clarify whether particular clinical characteristics predict the outcome of mild events. Evidence in the literature regarding chronic obstructive lung disease suggests that early aggressive treatment of pulmonary exacerbations in that disease improve the longer term clinical outcome;35 this could also be the case in CF. Recent observational data suggest that patients in US CF care centres in the highest quartile of lung function had more courses of intravenous antibiotics than those in the lowest quartile of lung function, suggesting a link between aggressive care and outcome.31 To explore fully this treatment approach in CF, it is necessary to be able to identify mild or early CF pulmonary exacerbations.
More recent data have been published regarding severe pulmonary exacerbations using variable definitions. Ellaffi and colleagues36 have recently presented data on 1 year outcomes for severe CF exacerbations in 69 patients, 29 of whom were admitted to the ICU. Overall 1 year survival in subjects admitted to the ICU was 52%. This figure concurs with other work in which the 1 year survival of CF patients admitted to ICUs at six university hospitals in Paris was 57%; only 14% (6/42 adults) of the cohort died during their first ICU admission.37 Multivariate predictors of mortality included annual decline in forced expiratory volume in 1 s (FEV1), simplified acute physiology score II (SAPS II) and the use of invasive mechanical ventilation. Improved outcomes have also recently been noted using non-invasive oxygen and bi-level ventilation.38 Given advances in non-invasive ventilation, these mortality rates are better overall than those reported in the 1990s when ICU mortalities (rather than 1 year mortality) ranged from 32% to 55%.39,40 Evidence regarding invasive mechanical ventilation for patients with severe pulmonary exacerbations is limited but generally points to a poor outcome, particularly in adults.41
Pulmonary exacerbation rates
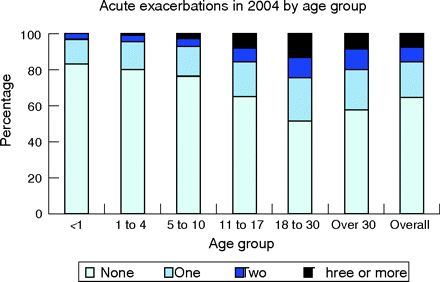
CF pulmonary exacerbation rates increase with age and more severe pulmonary impairment. Annual rates of CF pulmonary exacerbations by age and by FEV1 in the US CF Patient Registry are shown in figs 2⇓, 3A⇓ and 3B⇓. The pulmonary exacerbation rate was defined as a CF-related pulmonary condition requiring admission to hospital or use of home intravenous antibiotics. The percentage of patients with CF experiencing one or more pulmonary exacerbations per year rises when subjects become teenagers and young adults (fig 2⇓). Additional data from the Epidemiologic Study of Cystic Fibrosis noted an increasing annual rate at which patients with CF required intravenous antibiotics for pulmonary exacerbations with increasing age (increasing from 23% in subjects under age 6 years to 63% in those over the age of 18).34 Data from the US CF Patient Registry (fig 3A⇓ and B) delineate the relationship between lung function and pulmonary exacerbation rate. For adults the relationship is linear, with increasing exacerbation rates with decreasing lung function, while in children the relationship is non-linear (more consistent with an exponential fall in the exacerbation rate with higher FEV1). In both cases, better lung function, as measured by FEV1 percentage predicted, is associated with fewer pulmonary exacerbations.
Graph showing the proportion of patients with CF by age group in the US CF Registry population who experienced no exacerbations, one exacerbation, two exacerbations or three or more exacerbations during the year 2004. Data from Cystic Fibrosis Foundation Patient Registry, 2004 Annual Data Report to the Center Directors.83 © Cystic Fibrosis Foundation, 2005.
Mean annual rate of pulmonary exacerbation per decile of forced expiratory volume in 1 s (FEV1) percentage of predicted (each decile represents 10% of the total population) in (A) patients with CF <18 years of age and (B) patients ⩾18 years of age. The data represent the mean FEV1 percentage of predicted for 2004 in the US CF Registry in each decile of the population.
CF pulmonary exacerbation as a predictor and outcome variable
The annual rate of CF pulmonary exacerbations has clearly been associated with 2 year and 5 year survival in two separate prediction models evaluating the odds of death during follow-up.42–,45 CF pulmonary exacerbations requiring intravenous antibiotics have also been associated with later diminished lung function in children aged 1 to 6 years,46 with CF-related diabetes,47 with sleep disturbances and with health-related quality of life.48,49 It is an important marker of disease severity and, as such, has been used as an adjustment variable in studies of survival50–,52 and an important outcome measure when assessing the impact of socioeconomic status and environmental exposure on CF.53,54 The pulmonary exacerbation rate has also been used as an important variable to assess new outcome measures such as high resolution computed tomography of the chest or cough frequency.55,56 It will also be essential for assessing future outcome measures. The pulmonary exacerbation rate is clearly in the causal pathway of pulmonary decline in CF, thus linking an outcome to this rate strengthens its validity.
Microbiological epidemiology of a CF pulmonary exacerbation
Microbiological diagnosis
Chronic bacterial airway infections are characteristically seen in the majority of individuals with CF. These infections are commonly polymicrobial and rarely can be eradicated with antimicrobial treatment. “Polymicrobial” is defined as an individual patient at a particular point of time infected with a number of different organisms. Knowledge of the natural history of colonisation (culture positivity in the airway) and infection (culture positivity with an associated specific host serological response) can be helpful in the management of CF pulmonary exacerbations. However, the epidemiology can only be established with adequate diagnostic microbiology testing.
Culture of respiratory tract specimens from individuals with CF can present challenges to microbiology laboratories unaccustomed to processing them because of problems related to sample viscosity, the polymicrobial nature of infections and slow bacterial growth. In addition, many of the available commercial systems for organism identification and antimicrobial susceptibility testing are inaccurate for CF pathogens.57–,60 CF secretions are frequently very viscous, requiring special processing to sample the entire specimen adequately.61 This is probably due to a combination of the primary defect in CFTR, which results in dehydrated airway mucus, and the purulence resulting from airway inflammation. For this reason, CF samples may be treated with dithiothreitol, DNase, or another solubilising agent to decrease viscosity.
Polymicrobial infections are the norm in CF airway infections and can be problematic since the organisms in the specimen may have very different growth requirements. Pseudomonas aeruginosa is often present and, because of its mucoid phenotype, frequently overgrows both Gram-positive bacteria such as Staphylococcusaureus and more fastidious or slower growing Gram-negative organisms such as Haemophilus influenzae and Burkholderia cepacia complex. The use of selective media, which inhibit the growth of P aeruginosa, is very useful for the isolation of S aureus and H influenzae and is mandatory for the isolation of B cepacia complex (table 2⇓).61–,64 In addition, multiple subcultures may need to be performed to isolate pure bacterial cultures for identification and susceptibility testing. Slow bacterial growth also requires that culture plates receive prolonged incubation. Laboratories specialising in CF microbiology frequently use incubation times of 48 h for cultures expected to yield P aeruginosa, and up to 96 h before reporting a culture negative for B cepacia complex.63
Recommended culture conditions for CF pathogens from respiratory samples
Once isolated, organism identification may also be difficult, both because of the presence of a large number of unique bacteria and because of the phenotypic changes that even the more common organisms may undergo. The use of standard biochemical testing rather than commercial systems has been recommended for identification of Gram-negative non-fermenting bacteria.57,58 In addition, molecular techniques, especially polymerase chain reaction, have proved useful for bacterial identification, both directly in sputum and for isolated organisms growing in pure culture.65–,68
Microbiological sampling
Sampling of lower airway secretions is considered essential for determining the infectious aetiology of pulmonary exacerbations in CF. This is most readily accomplished using expectorated sputum. However, some individuals with CF are unable to expectorate. This is most common in young children. Bronchoalveolar lavage is an excellent way of sampling the lower airway in non-expectorating CF individuals, but this is too invasive for routine culturing.
Oropharyngeal swabs have served as a surrogate but may not be representative of lower airway infection.69,70 Rosenfeld and colleagues compared oropharyngeal swab cultures with simultaneous bronchoalveolar lavage cultures in 141 young children.70 For predicting growth of P aeruginosa from the lower airway in subjects aged 18 months or younger, oropharyngeal cultures had a sensitivity of 44% and a specificity of 95%. H influenzae was similar, but the specificity was significantly lower for S aureus. Oropharyngeal swabs obtained after chest physiotherapy were found to have increased sensitivity and specificity for the detection of both P aeruginosa and S aureus compared with swabs obtained before physiotherapy.71
Hypertonic saline induction of sputum has been reported to be a good surrogate for lower airway sampling in CF.72,73 Several studies suggest that induced sputum may be more sensitive in detecting bacteria in the lower airway than expectorated sputum and even bronchoalveolar lavage.73–,75 Sputum induction has been used to monitor both inflammation and infection after intravenous antibiotic treatment for pulmonary exacerbations in CF.23
Organisms
Chronic CF airway infections are commonly caused by one or more of a characteristic set of bacterial pathogens that appear to be acquired in an age-dependent sequence (fig 4⇓). Of the most frequently identified bacterial organisms causing airway infections in CF, only S aureus is generally considered to be pathogenic in individuals who are not immunocompromised. P aeruginosa, B cepacia complex, non-typeable H influenzae, Stenotrophomonas maltophilia, and Achromobacter xylosoxidans are often considered opportunistic pathogens. All of these organisms can variably be associated with pulmonary exacerbations in CF. Other chronically infecting organisms seen in CF that are also generally non-pathogenic in the healthy host include Aspergillus and non-tuberculous mycobacteria. These organisms are usually not associated with exacerbations in patients with CF.
Prevalence of selected respiratory pathogens in respiratory cultures of CF patients by age. Data from Cystic Fibrosis Foundation Patient Registry, 2004 Annual Data Report to the Center Directors.83 © Cystic Fibrosis Foundation, 2005.
Early infections in CF airways are most frequently caused by S aureus and H influenzae, organisms that may be seen in other young children with chronic illnesses and in adults with non-CF bronchiectasis. S aureus is often the first organism cultured from young children with CF.76 However, there continues to be debate about the significance of S aureus in the pathogenesis of CF lung infection.77 Historically, significant improvements in patient longevity have been associated with the advent of anti-staphylococcal therapy.78 However, several published studies of the efficacy of prophylactic anti-staphylococcal antibiotics have not demonstrated clinical improvement in the treated populations.79,80
Non-typeable H influenzae is also isolated from the respiratory tract early in the course of CF. In natural history studies of prospectively followed infants with CF, including those identified by neonatal screening, H influenzae was the most common organism isolated from lower airway cultures in the first year of life.69,81 Although H influenzae is associated with exacerbations in patients with non-CF bronchiectasis,82 its role in progressive airway infection and inflammation in patients with CF is unclear.
P aeruginosa is by far the most significant pathogen in CF. Up to 80% of patients with CF are eventually infected with P aeruginosa,83 and acquisition of Pseudomonas, particularly organisms producing mucoid exopolysaccharide, is associated with clinical deterioration.46,84–,87P aeruginosa isolates from the lungs of patients with CF are phenotypically quite distinctive. These characteristics—including mucoidy, lipopolysaccharide changes (loss of O-side chains, distinctive acylation), loss of flagella-dependent motility, increased auxotrophy, and antibiotic resistance—are not present in isolates causing initial colonisation.88–,92 Early isolates look much like environmental isolates in their phenotype. Phenotypic changes appear to be selected within the CF airways and are more frequent when patients have been infected for a prolonged period of time. The ability to form biofilms may also be increased in chronic P aeruginosa isolates from individuals with CF.93
Several studies have demonstrated multiple genotypes of P aeruginosa present in a single sputum sample. Thus, Aaron and colleagues (as noted above) investigated how commonly acquisition of a new genotype of P aeruginosa was associated with a CF exacerbation.18 Among 80 individuals followed for 2 years with quarterly sputum cultures, 40 patients experienced a pulmonary exacerbation. Only 36 had isolates that could be genotyped and, of those, only two subjects demonstrated acquisition during exacerbation of a new clone that had not been present during a period of clinical stability.
Bacterial pathogens that are identified later in the course of CF airways disease include B cepacia complex, S maltophilia, and A xylosoxidans. Of these, B cepacia complex is the most serious because of its association with rapid progression to severe necrotising pneumonia and death.94,95 At least 10 distinctive genomovars of B cepacia have been identified and several have been named as distinct species (LiPuma, personal communication).96 The genomovars most commonly associated with CF airway infections in Europe, North America and Australia include B cepacia (genomovar I), B multivorans (genomovar II), B cenocepacia (genomovar III), B stabilis (genomovar IV), B vietnamiensis (genomovar V), B dolosa (genomovar VI), and B ambifaria (genomovar VII).97,98,99,100 The vast majority of CF airway infections with B cepacia complex are caused by B multivorans (genomovars II), B cenocepacia (genomovar III) and B vietnamiensis (genomovar V).97 Although there are exceptions, most dramatically B dolosa, most of the severe infections and those associated with epidemic spread have been from B cenocepacia.101,102 Several clonal lineages are distributed widely across Europe and North America.97,101
S maltophilia and A xylosoxidans are seen more commonly than B cepacia complex in CF patients with advanced lung disease, but are generally less virulent. Several epidemiological studies examining their association with morbidity and mortality in CF have not shown a correlation between infection and outcome.50,103
Antibiotic resistance
Susceptibility testing of CF isolates of P aeruginosa is difficult for many of the same reasons that affect organism isolation and identification. Slow growth and mucoidy may affect the usefulness of automated systems for susceptibility testing of P aeruginosa as well as for organism identification.59,60 When compared with broth microdilution, agar diffusion methods including disc diffusion (Kirby-Bauer) and Etest performed well for most of the antibiotics tested.104
Early infections with P aeruginosa are commonly susceptible to anti-pseudomonal β-lactam antibiotics (including ticarcillin, piperacillin, ceftazidime, cefoperazone, and the carbopenems), the aminoglycosides and the fluoroquinolones. However, as patients age, antibiotic resistance appears more frequently. At Danish CF centres a significant increase in resistance to β-lactams was seen over two decades, but no correlation was found between the increase in minimal inhibitory concentration and the number of courses of anti-pseudomonal treatment.105 Multiple antibiotic resistance, defined as in vitro susceptibility to only a single class of antimicrobial agents, has been reported in up to 11.6% of P aeruginosa isolates from individuals with CF in the USA.64 Data from Italian CF centres found that 17.4% of P aeruginosa isolates were multiply resistant.106 These multiply resistant isolates present an important management problem to clinicians. In order to help identify active antibiotic treatment for patients with multiresistant isolates, non-standard methods of susceptibility including synergy testing and multiple combination bactericidal testing (MCBT) have been developed to test two and three drug combinations.107,108 These methods are being used by CF clinicians to guide treatment. However, a recent prospective study examining the clinical efficacy of MCBT for directing antibiotic treatment in 132 CF subjects with pulmonary exacerbations did not result in improved clinical or microbiological outcomes.109 Interestingly, even standard susceptibility testing has not been clearly shown to improve patient outcome.110 More recently, evidence of biofilm formation by organisms in the CF airway has prompted the investigation of biofilm susceptibility testing.111,112 Different drugs and drug combinations appear to be efficacious against P aeruginosa growing in biofilms; this may help to explain non-bactericidal mechanisms of activity of antimicrobial treatment.
While clinical laboratories have not been routinely looking for methicillin resistance in S aureus isolated from patients with CF, a survey of isolates from a number of CF centres in the USA suggested that the rate of resistance in CF is comparable to that in the general population.64 Recent data from the US CF Foundation found that 14.6% of S aureus was methicillin resistant.83 Vancomycin tolerance and resistance have both been described in human isolates of S aureus,113 and it is likely that they will also be seen in individuals with CF as they become more common.
B cepacia complex organisms are often highly antibiotic resistant. All are intrinsically resistant to the aminoglycosides114 and the rate of in vitro resistance to the β-lactam antibiotics, with the exception of meropenem, is also quite high.115,116 The quinolones have variable activity, but resistance can be readily induced.115 In vitro susceptibility testing suggests that there are combinations of antibiotics that act synergistically against B cepacia complex using either synergy testing or MBCT.116,117 Synergy testing, using two drug combinations, found that no active combination could be identified for 57% of isolates tested.117 The most active combinations were chloramphenicol plus minocycline (49% of isolates) and chloramphenicol plus ceftazidime (26% of isolates). MBCT testing using two or three drug combinations determined that at least one combination could be identified for all isolates tested.108 The majority of active combinations included meropenem. Unfortunately, it was not possible to predict for a given isolate whether a drug combination would be synergistic, additive or antagonistic.
Other antibiotic resistant Gram-negative CF isolates include S maltophilia and A xylosoxidans. Treatment of these organisms is often complicated by resistance to the aminoglycosides and variable susceptibility to the β-lactams and quinolones. The most active single drugs in vitro against S maltophilia are ticarcillin/clavulanate and trimethoprim/sulfamethoxazole; the most active combination in synergy studies is ticarcillin/clavulanate plus aztreonam.118 In a study of 106 CF isolates of A xylosoxidans, the most active drugs were imipenem (59% susceptible), piperacillin/tazobactam (55%), meropenem (51%) and minocycline (51%).119 The most active additive or synergistic combinations were chloramphenicol plus minocycline, ciprofloxacin plus imipenem, and ciprofloxacin plus meropenem.
CONCLUSIONS
Research regarding pulmonary exacerbations in cystic fibrosis continues to evolve, generating new hypotheses regarding the pathophysiology of CF and improving our understanding of the natural history of the disease in patients with CF. Pulmonary exacerbations continue to have a significant impact on the lives of children and adults with CF. Improving our understanding of these events will have implications for basic research and clinical research in CF. Although much has been learned about pulmonary exacerbations in CF, the most problematic areas of research continue to be our limited understanding of the basic pathophysiology of these events and the need for clear consensus diagnostic criteria.
REFERENCES
Footnotes
Support: Leroy Matthew Physician Scientist, Cystic Fibrosis Foundation; NIH (NHBLI) 1 K23 HL72017-01 (CG).
Competing interests: None.
Linked Articles
- Airwaves
- Editorials