Article Text
Abstract
Background House dust mite (HDM) allergens have been reported to increase airway epithelial permeability, thereby facilitating access of allergens and allergic sensitisation.
Objectives The authors aimed to understand which biochemical properties of HDM are critical for epithelial immune and barrier responses as well as T helper 2-driven experimental asthma in vivo.
Methods Three commercially available HDM extracts were analysed for endotoxin levels, protease and chitinase activities and effects on transepithelial resistance, junctional proteins and pro-inflammatory cytokine release in the bronchial epithelial cell line 16HBE and normal human bronchial cells. Furthermore, the effects on epithelial remodelling and airway inflammation were investigated in a mouse model.
Results The different HDM extracts varied extensively in their biochemical properties and induced divergent responses in vitro and in vivo. Importantly, the Greer extract, with the lowest serine protease activity, induced the most pronounced effects on epithelial barrier function and CCL20 release in vitro. In vivo, this extract induced the most profound epithelial E-cadherin delocalisation and increase in CCL20, CCL17 and interleukin 5 levels, accompanied by the most pronounced induction of HDM-specific IgE, goblet cell hyperplasia, eosinophilic inflammation and airway hyper-reactivity.
Conclusions This study shows the ability of HDM extracts to alter epithelial immune and barrier responses is related to allergic sensitisation but independent of serine/cysteine protease activity.
- Protease
- bronchial epithelium
- Th2-mediated inflammation and asthma
- airway epithelium
- allergic lung disease
- asthma
- asthma genetics
- asthma mechanisms
- COPD mechanisms
- innate immunity
- lymphocyte biology
- COPD exacerbations
Statistics from Altmetric.com
- Protease
- bronchial epithelium
- Th2-mediated inflammation and asthma
- airway epithelium
- allergic lung disease
- asthma
- asthma genetics
- asthma mechanisms
- COPD mechanisms
- innate immunity
- lymphocyte biology
- COPD exacerbations
Key messages
What is the key question?
The proteolytic activity of house dust mite allergens was hypothesised to be crucial for epithelial barrier dysfunction and subsequent activation of the innate immune response in asthma.
What is the bottom line?
This study determined that the divergent abilities of these extracts to alter epithelial barrier and immune function in vitro are uniquely associated with the capacity to induce allergic sensitisation and asthma phenotypes in vivo.
Why read on?
This makes the epithelial barrier an important target for future therapeutic strategies in asthma.
Introduction
Allergic asthma is characterised by allergen-specific IgE, T helper 2 (Th2)-mediated airway inflammation, airway remodelling and airway hyper-reactivity (AHR). The airway epithelium forms the first structural barrier against inhaled allergens. This epithelial barrier function is maintained by the formation of tight junctions (TJs), composed of zonula occludens (ZO) 1–3, occludin and claudins 1–5, as well as adherens junctions (AJs), consisting of E-cadherin, β-catenin and α-catenin. Whereas TJs largely contribute to epithelial impermeability, E-cadherin is thought to provide the architecture required to form TJs.1
Many aeroallergens, including house dust mite (HDM), fungi and cockroach contain proteolytic activities.2 3 The HDM allergens Dermatophagoides pteronyssinus (Der p) 1, 3, 6 and 9 are cysteine and/or serine proteases, of which the serine peptidase activity has previously been reported to cleave ZO-1, occludin and to a lesser extent also E-cadherin.4 In addition to direct cleavage of junctional proteins, serine proteases can activate protease-activated receptor (PAR)-2, which can induce disruption of E-cadherin mediated cell–cell contacts.5 This may facilitate access of allergens to submucosal cells and promote allergic inflammation.6 7 In support of this notion, we have previously demonstrated that downregulation of E-cadherin in bronchial epithelium increases expression of the pro-allergic factors CCL17 and thymic stromal lymphopoietin (TSLP), which attract Th2 cells and promote Th2 cell differentiation respectively.8 9 Furthermore, PAR-2 activation by serine proteases induces activity of intracellular signalling pathways, including nuclear factor-κB (NF-κB), and subsequent release of the pro-inflammatory cytokines interleukin (IL)-6, IL-8, granulocyte macrophage colony-stimulating factor (GM-CSF) and TSLP in airway epithelium in vitro and in vivo.10 11 Based on studies in mouse models of asthma, the presence of proteases in HDM and subsequent PAR-2 activation are thought to play an important role in allergic sensitisation.12–14 In addition, a number of other biochemical activities and components of HDM, including chitin/chitinases, ß-glucan and lipopolysaccharide (LPS), may contribute to allergic sensitisation.15–17
We hypothesised that proteolytic activity of HDM allergens is crucial for epithelial barrier dysfunction and subsequent activation of the innate immune response in asthma. We investigated different HDM extracts that vary extensively in biochemical properties and proteolytic activities, and assessed their effects on epithelial barrier function, release of pro-inflammatory cytokines and induction of Th2 responses, in vitro and in vivo. We demonstrate that the divergent abilities of these extracts to alter epithelial barrier and immune function in vitro are uniquely associated with the capacity to induce allergic sensitisation and asthma phenotypes in vivo. Of interest, this appeared to be independent of serine protease activity.
Methods
See the online supplement for additional details.
HDM extracts
Three whole crushed body mite extracts were used. The first was kindly provided by Citeq Biologics (Groningen, The Netherlands), the other two were purchased from ALK-Abello (Abello, Spain) and from Greer Laboratories (Lenoir, North Carolina, USA). When indicated, HDM extracts were pretreated with the serine inhibitor 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) (Sigma, St Louis, Missouri, USA) or the cysteine inhibitor E-64 (Sigma) at concentrations of 0.1 mM and 0.01 mM, respectively, for 30 min at 37°C, or heat inactivated for 1 h at 95°C.
Cells
The human bronchial epithelial cell line 16HBE14o- was kindly provided by Dr D C Gruenert (University of California, San Francisco, California, USA). Normal human bronchial epithelial (NHBE) cells were derived from Lonza (Walkersville, Maryland, USA). The cells were cultured as previously described8 18 and used as indicated for electric cell-substrate impedance sensing (ECIS), ELISA, immunodetection and immunofluorescent staining (see online data supplement).
Electric cell-substrate impedance sensing
Electrical resistance was measured using ECIS in a confluent monolayer of 16HBE cells, as previously described.19 20 The ECIS is a technique that allows for real-time quantitative monitoring of changes in resistance as measurement of cell–cell contacts and changes in capacitance as measurement of cell–matrix contacts.19 Resistance and capacitance to current flow were measured at frequencies of 400 Hz and 40 kHz respectively (Applied Biophysics, Troy, New York, USA).
Animals
Male Balb/c mice (6–8 weeks) were purchased from Charles River Laboratories (L'Arbresle Cedex, France), kept under specific pathogen-free conditions and maintained on a 12 h light–dark cycle, with food and water ad libitum. Experiments were approved by the Institutional Animal Care and Use Committee of the University of Groningen (The Netherlands).
HDM sensitisation protocol
HDM extracts and LPS (Sigma) were dissolved in sterile phosphate-buffered saline (PBS; 2.5 mg total weight/ml) and administered intranasally in 10 μl, twice weekly for 5 weeks. Mice were anaesthetised with isoflurane/oxygen (Nicholas Piramal India Ltd, London, UK). Twenty-four hours after the last sensitisation airway responsiveness was measured by Flexivent (SCIREQ, Montreal, Canada), lungs were lavaged and blood and lung tissues were collected.
Cytokine assay in cell supernatants and mouse lung tissue
Human CCL20 and GM-CSF protein was measured in cell-free supernatants from 16HBE cells and murine IL-5, IL-13, CCL20, thymus and activation-related chemokine (TARC), TSLP and GM-CSF was determined in cell-free supernatant from homogenised lung using Duoset ELISA Development Kit (R&D Systems, Minneapolis, Minnesota, USA). The ELISAs were used according to the manufacturer's guidelines.
Statistical analysis
We assumed normal distribution and used the Student t test for paired observations in the experiments with 16HBE. In animal experiments, the Mann–Whitney U test was used.
Results
Disruption of bronchial epithelial cell–cell contacts upon exposure to the HDM extracts
First, we tested the HDM extracts for proteolytic activity, chitinase levels and endotoxin content. Although we stratified the extracts on the basis of total proteolytic activity, analyses revealed that the extracts varied extensively in their other biochemical properties, including protein content (table 1). The Citeq and ALK extract contained the highest serine/cysteine protease activities, while levels of these proteases were relatively low in the Greer extract. Instead, this extract contained other, partially heat-insensitive proteases and the highest Der p2 content. ALK and Greer comprised substantial heat-sensitive exochitinase and endochitinase activity (table 1).
Biochemical properties of the house dust mite (HDM) extracts in the concentration applied in vitro
To test our hypothesis that the proteolytic activity of the HDM extracts is critical for airway epithelial barrier dysfunction, we exposed 16HBE cells to the different extracts in concentrations rendering equal levels of total proteolytic activity (see table 1). First, we evaluated the effect on occludin protein stability, since Der p1 has been shown to induce cleavage of occludin, leading to increased permeability of the epithelial layer.7 Exposure to all HDM extracts induced smaller molecular weight cleavage products of ∼37 kD and ∼25 kD, as shown by immunodetection (figure 1A), although the latter fragment was not observed with the Citeq extract. A similar degradation pattern was previously described by Wan et al, when epithelial cells were exposed to Der p1.7 Furthermore, the Greer extract yielded an additional fragment of ∼45 kD, which was also observed upon exposure to a protease cocktail used as positive control. Importantly, the appearance of degradation fragments could not be blocked by treatment of the extracts with serine protease inhibitor AEBSF, cysteine protease inhibitor E64 or heat inactivation (figure 1A), suggesting that these effects of HDM can occur independently of serine/cysteine protease or chitinase activity.
Effects of the house dust mite (HDM) extracts on tight and adherens junctions in human bronchial epithelium. 16HBE cells were seeded in duplicates, grown for 3–5 days in 24-well plates, LabTeks or electric cell-substrate impedance sensing arrays, serum deprived overnight and incubated with or without a protease cocktail (PC; 2.5 μg/ml) or HDM extracts that were treated with or without 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) (0.1 mM), E64 (0.01 mM) or heat inactivated (HI) (95°C, 1 h). (A) Total cell lysates were prepared and occludin was detected by immunoblotting. ß-actin was used as a control (con) for equal loading. A representative from three independent experiments is shown. (B) Resistance was measured immediately after HDM administration at 400 Hz and normalised to the values prior to HDM administration. Mean levels (±SEM) are shown (n=3). (C) Resistance was measured immediately after HDM administration at 400 Hz and normalised to the values prior to HDM administration, compared with AEBSF/E64-treated or heat-inactivated HDM. Mean levels (±SEM) are shown (n=3). **p<0.01 between control and Greer-treated 16HBE cells, #p<0.001 between control and heat-inactivated Greer-treated 16HBE cells, and +p<0.05 between control and AEBSF/E64-treated Greer-treated 16HBE cells. (D) E-cadherin, zonula occludens (ZO)-1 and occludin were detected 15 min after stimulation with or without (control) HDM in 16HBE cells by immunofluorescent staining. Representatives from three independent experiments are shown. (E) E-cadherin, ZO-1 and occludin were detected 15 min after stimulation with or without (control) HDM in normal human bronchial epithelial cells by immunofluorescent staining. Representatives from three independent experiments are shown.
To directly determine the effect of HDM on epithelial barrier function, we measured electrical resistance of 16HBE cell monolayers using ECIS. Exposure to the Greer, but not Citeq or ALK, extract induced a transient fall in epithelial resistance, with a maximum effect at ∼10–20 min and recovery to baseline values within 60 min (figure 1B). This effect could not be inhibited by heat inactivation or pretreatment of the extract with the serine/cysteine protease inhibitors (figure 1C).
Prolonged exposure (24 h) to the Citeq extract, but not the other HDM extracts, dramatically decreased epithelial resistance (online supplementary figure 2A), which was parallelled by detachment of the cells (online supplementary figure 2B) and attenuated by treatment of the extract with the serine protease inhibitor (data not shown). LPS, which was present in our extracts (see table 1), did not affect epithelial barrier function at the highest concentration found in the extracts (50 U/ml; data not shown).
Next, we visualised the effects of the HDM extracts on TJ and AJ integrity. Using immunofluorescent staining, we observed continuous circumferential localisation of E-cadherin, ZO-1 and occludin at the cell membrane of the 16HBE cells at baseline conditions (figure 1D). Exposure to all three HDM extracts (15 min) induced delocalisation of E-cadherin, ZO-1 and occludin from the membrane. In line with the Greer-induced epithelial barrier dysfunction, these effects were most pronounced upon exposure to the Greer extract (figure 1D). In accordance with the effects on occludin cleavage (figure 1A), heat inactivation of the HDM extracts did not abrogate the delocalisation of occludin, ZO-1 and E-cadherin (online supplementary figure 1). To verify our results in primary cells, we also studied the effects of the HDM extracts on NHBE cells. Here, all HDM extracts induced marked delocalisation of E-cadherin, ZO-1 and occludin (figure 1E). Again, the Greer extract induced the most pronounced effect on E-cadherin and occludin, although this was not clearly the case for ZO-1.
In summary, all extracts induced delocalisation of junctional proteins to some extent, with the most pronounced effect of the Greer extract, which also induced a transient decrease in epithelial resistance in 16HBE cells.
Cytokine levels upon exposure of bronchial epithelial cells to the HDM extracts
HDM can induce epithelial expression of pro-inflammatory cytokines and chemokines, including CCL20 and GM-CSF.10 17 21 CCL20 is known to attract naïve dendritic cells towards the airway mucosa,22 while GM-CSF induces maturation and activation of these cells.23 The Greer extract, but not the Citeq or ALK extracts, induced a strong and significant increase (∼fourfold) in CCL20 levels in 16HBE cells, which was not significantly affected by heat treatment of the extract (figure 2A). The secretion of GM-CSF was slightly, but not significantly increased upon exposure to all extracts, and again not affected upon heat inactivation (figure 2B). Furthermore, LPS (50 EU/ml) did not affect CCL20 or GM-CSF secretion by 16HBE cells (data not shown). Additional experiments with NHBE cells showed that both the Citeq and Greer extract induced a significant increase in CCL20 levels, while GM-CSF levels were also significantly enhanced after exposure to the Greer extract (figure 2C,D).
Pro-inflammatory cytokine production upon exposure of 16HBE cells to the house dust mite (HDM) extracts. Cells were seeded in duplicates, grown to confluence, serum deprived overnight and left unstimulated (control, con) or stimulated with heat-inactivated (HI) or untreated HDM extracts. Cytokines were measured in cell-free supernatants. (A) Levels of CCL20 after 24 h of stimulation with or without HDM extract in 16HBE cells. (B) Levels of granulocyte macrophage colony-stimulating factor (GM-CSF) after 48 h of stimulation with or without HDM extract in 16HBE cells. (C) Levels of CCL20 24 h with and without HDM extract in normal human bronchial epithelial (NHBE) cells. (D) Levels of GM-CSF 24 h after stimulation with and without HDM extract in NHBE cells. Mean absolute levels (±SEM) are shown (n=3). *p<0.05 between control and HDM-treated 16HBE cells.
In vivo responses to the HDM mite extracts
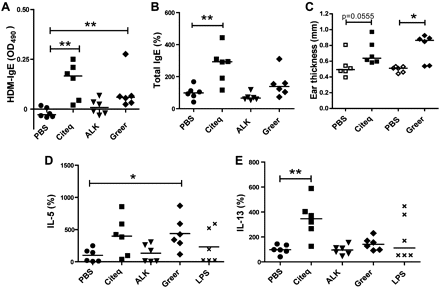
Next, we tested which of the HDM extracts was able to induce airway inflammation in vivo. Here, mice received 10 μl of 2.5 mg/ml HDM extract (see table 2) or 10 μl PBS at each administration. Of note, HDM extracts were administered based on total weight and not on protein content or on total protease content (as in the in vitro experiments). Administration of all HDM extracts induced delocalisation of E-cadherin in airway epithelium compared with the PBS-treated mice, with the most pronounced effect of the Greer extract (figure 3A,B). Interestingly, these effects could already be observed after a single administration of the HDM extracts to naïve mice (data not shown). Importantly, the Greer extract, but none of the other extracts, also induced substantial goblet cell hyperplasia (figure 3A).
Biochemical properties of the house dust mite (HDM) extracts in the concentration applied in vivo
Effect of the house dust mite (HDM) extracts in a mouse model of asthma. Balb/c mice (n=6–14 per group) were exposed to 10 μl of different HDM extracts (2.5 mg/ml), lipopolysaccharide (LPS) (2.5 mg/ml) or phosphate-buffered saline (PBS) twice a week for 5 weeks. Mice were sacrificed 24 h after the final intranasal challenge. Lung sections were stained for (A) E-cadherin and peroxidase-acid Schiff (PAS). Representative pictures are shown (original magnification ×40). (B) Measurement of E-cadherin positive membrane staining (%) analysed by Image-Pro Plus. Levels were expressed as percentage of E-cadherin staining on the membrane of the airway epithelium, medians are shown. ELISA measurements of (C) CCL20, (D) CCL17, (E) thymic stromal lymphopoietin and (F) granulocyte macrophage colony-stimulating factor (GM-CSF) in homogenised lung tissue, 24 h after the final intranasal challenge. Values were normalised to total protein content and expressed as percentages of control values. Relative levels and medians are shown. *p<0.05, **p<0.01 and ***p<0.001 between HDM-treated and PBS-treated mice.
Interestingly, Greer extract-treated mice, but not mice treated with the other extracts, displayed significantly increased levels of CCL20 and CCL17 in lung tissue compared with PBS-treated mice (figure 3C,D). None of the HDM extracts significantly altered TSLP or GM-CSF levels 24 h after the last application (figure 3E,F). Furthermore, lung cytokine/chemokine levels were not significantly altered upon administration of an equivalent amount of LPS (figure 3C–F; see also table 3 in the online supplement for absolute values). In addition, Eotaxin-1 and KC levels were increased after exposure to Greer and to a lesser extent also to the Citeq extract, while exposure to the Citeq extract also increased the levels of IL-17 (online supplementary figure 3).
To assess whether mucosal application of the different HDM extracts induced allergic sensitisation in vivo, we analysed the HDM-specific IgE responses. Both the Citeq and Greer, but not ALK, extract, induced a significant increase in HDM-specific IgE levels (figure 4A), while only the Citeq extract significantly increased total IgE levels (figure 4B, see also table 3 in the online supplement for absolute values). To confirm that HDM-specific IgE levels were sufficient to induce an immediate allergic response, we measured the ear swelling response 2 h after local HDM injection in the HDM-treated mice. We observed a significant increase in ear thickness in Greer extract-treated mice (figure 4C) and a trend in Citeq extract-treated mice (p=0.055), indicating that both HDM extracts were able to induce allergic sensitisation via the airways.
The allergic sensitisation response after house dust mite (HDM) exposure. Balb/c mice (n=6–8 per group) were exposed to 10 μl HDM extracts (2.5 mg/ml), lipopolysaccharide (LPS) (2.5 mg/ml) or phosphate-buffered saline (PBS) twice a week for 5 weeks. (A) ELISA measurements of HDM-specific IgE, shown as absolute values. (B) Total IgE in mouse serum. Levels are expressed as percentages of control values and medians are shown. (C) IgE-dependent immediate allergic response measured by ear thickness (mm) after intracutaneous injection of 25 μg/ml HDM in the right ear and PBS as control in the left ear. Absolute values and medians are shown. (D) Interleukin (IL)-5 levels in homogenised lung tissue, 24 h after the final intranasal challenge. Values are normalised to total protein content and expressed as percentages of control values. Relative levels and medians are shown. (E) IL-13 levels in homogenised lung tissue, 24 h after the final intranasal challenge. Values are normalised to total protein content and expressed as percentages of control values. Relative levels and medians are shown. *p<0.05 and **p<0.01 on comparison between HDM-treated and PBS-treated mice.
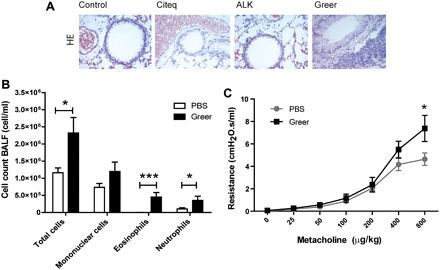
Additionally, treatment with the Greer extract induced a significant increase in lung IL-5 (figure 4D), while the Citeq extract induced increased levels of IL-13 (figure 4E; see also table 3 in the online supplement for absolute values). Treatment with the ALK extract or LPS did not induce a significant increase in these Th2 cytokines (figure 4D,E). In accordance with the increase in IL-5, exposure to the Greer extract, but none of the other extracts, induced lung inflammatory cell recruitment as evaluated by haematoxylin eosin staining (figure 5A). Quantification of the profile of the inflammatory cells in bronchoalveolar lavage (BAL) of Greer-extract treated mice revealed that the increased eotaxin and KC levels were indeed accompanied by increased numbers of eosinophils and neutrophils (figure 5B). Since the Greer extract was the only extract to induce airway inflammation, we aimed to confirm that this HDM extract was also able to induce AHR. As shown in figure 5C, the Greer extract induced an increase in AHR to metacholine compared with PBS-treated control mice, which only reached significance at the highest dose of metacholine.
The inflammatory response after allergen exposure. Balb/c mice (n=6–8 per group) were exposed to 10 μl phosphate-buffered saline (PBS), Citeq, ALK and/or Greer extract as indicated (2.5 mg/ml) twice a week for 5 weeks. Mice were sacrificed 24 h after the final intranasal challenge. (A) Lung sections were stained for haematoxylin eosin. Representative pictures are shown (magnification ×40). (B) Total, mononuclear, eosinophiland neutrophil numbers were determined in bronchial airway lavage fluid (BALF). Absolute numbers and medians are shown. (C) Airway hyperreactivity was measured by Flexivent. Absolute mean values (±SEM) are shown. *p<0.05 and ***p<0.001 on comparison between HDM-treated and PBS-treated mice.
In summary, the extract that exerted the most pronounced effects on epithelial immune and barrier function in vitro and on epithelial remodelling in vivo also induced the most profound allergic responses in our mouse model.
Discussion
The airway epithelial barrier is an important target for the proteolytic activities of allergens and may play a crucial role in allergic sensitisation.24 Disruption of the epithelial barrier may facilitate transport of allergens to allergen-presenting cells and promote pro-inflammatory activities of the epithelium.14 21 25 In this study we investigated the effects of three different HDM extracts, varying extensively in composition and proteolytic activities. Interestingly, the Greer extract, which exerted the most pronounced effects on epithelial immune and barrier function in vitro, also induced allergic sensitisation and manifestations of asthma, including goblet cell hyperplasia, inflammatory cell infiltrates and increased Th2 cytokine levels in vivo. Importantly, this extract displayed the lowest serine and cysteine protease activity. Furthermore, the in vitro effects could not be prevented by heat inactivation and could not be mimicked by LPS administration. In line with our observations, De Alba et al have shown that the Greer extract was still able to induce manifestations of asthma upon heat inactivation in a rat model.26 Our data show for the first time that serine/cysteine proteases and chitinases in HDM extracts are not critically required for disruption of epithelial barrier function in vitro and innate immune responses in vivo, and hence subsequent allergic sensitisation and eosinophilic airway inflammation.
The Greer and Citeq extracts, which displayed the lowest and the highest serine protease activity respectively, induced HDM-specific IgE levels and an immediate allergic response in vivo, as measured by ear swelling upon topical application of the allergen. These data indicate that an IgE response can be induced independently of serine protease activity, in line with a previous report in which mice were sensitised by intratracheal aspiration with either protease-active or protease-depleted German cockroach faeces extract.27 Remarkably, the same group reported that protease activity did have an effect on serum IgE when the same allergen extracts were precipitated on alum and applied intraperitoneally,3 indicating that the relevance of the protease activity for the induction of an IgE response might depend on the context in which the allergen is presented. The Citeq extract also induced an increase in IL-13 and IL-17 in the lungs, which might contribute to the IgE response induced by Citeq.28 Importantly, we observed that treatment with the Greer extract, containing the lowest serine protease activity, increased HDM-specific IgE levels and induced goblet cell hyperplasia, delocalisation of E-cadherin, profound (eosinophilic) airway inflammation, AHR and increased KC, CCL17, Eotaxin-1 and IL-5 levels in the lungs. In contrast, the Greer extract did not induce substantial secretion of IL-17, indicating that the KC production induced by this extract is likely responsible for recruitment of the neutrophils into the BAL. The widely divergent responses induced by the three different HDM extracts reflect the remarkable differences in biochemical composition between the extracts (see table 1), precluding a straightforward association of their individual properties to the induction of a defined biological response in vitro or in vivo. However, we observed a very interesting and highly relevant positive association between several biological responses induced by a single HDM extract. The Greer extract induced loss of barrier function and pro-inflammatory responses in vitro, and allergic sensitisation, airway remodelling, AHR and eosinophilic inflammation in vivo, suggesting a putative causal relationship between airway epithelial responses and the induction of a Th2-polarised immune response.
The induction of the above-mentioned asthma manifestations appears to be independent of serine/cysteine proteases, chitinase activities and LPS levels. Our data show that these manifestations are induced by the Greer extract that uniquely decreased epithelial barrier function and induced the most profound delocalisation of occludin, ZO-1 and E-cadherin, an effect that is independent of heat inactivation of the extract. Furthermore, the Greer extract was still proteolytically active and able to cleave occludin upon heat inactivation. Thus, an unidentified heat-insensitive protease might contribute to the disruption of epithelial TJ proteins. The intracellular protein ZO-1 and the more basolaterally positioned E-cadherin were also delocalised upon exposure to all HDM extracts, indicating involvement of intracellular processes, for instance activation of PAR-2 receptors,5 known to be induced by serine proteases. Alternatively, activation of pattern recognition receptors may indirectly disrupt epithelial junctions. HDM extracts contain microbiological glucose structures, for example β-glucan, which can activate epithelial C-type lectin receptors, including dectin-1.17 Activation of these receptors induces Ca2+ fluxes29 and we speculate that this may lead to cleavage of cell–cell contact proteins by activation of the endogenous protease calpain.30 It has also been described that activation of the dectin-1 receptor induces rapid secretion of CCL20 by 16HBE cells.17 We only observed a substantial increase in CCL20 levels in vitro and in vivo upon exposure to the Greer extract. Thus, it will be of interest to further study the role of β-glucan in the effects of HDM extracts on epithelial immune barrier function.
Next to proteolytic allergens, HDM contains Der p2, a non-proteolytic allergen, which is structurally homologue to protein associated with the TLR4ecto-domain (MD-2). This enables its interaction with toll-like receptor-4 (TLR4), which may facilitate airway inflammation.31 Interestingly, the Greer extract with the most pronounced effects on epithelial barrier function contained the highest Der p2 levels. However, previously the effects of Der p2 on bronchial epithelial cells in vitro have been shown to disappear upon heat inactivation,32 whereas our observed effects remained upon heat treatment. Thus a role for Der p2 in the observed effects seems unlikely. In addition to the potential activation of TLR4 by Der p2, biochemical analysis revealed that all three HDM extracts contained substantial levels of LPS, which can also activate TLR4 (see table 1). However, LPS exposure did not mimic the effects on barrier function in vitro or inflammatory responses in vivo, although we cannot exclude a role for a synergistic interaction between TLR4 and PAR-2. In addition, the LPS content in the HDM extracts did not correlate with neutrophil recruitment, although this might have been expected.33 Finally, chitins as well as the activity of chitinases present in allergen extracts have been suggested to play a role in asthma.15 The effects observed in our study are not likely due to chitinases, since chitinase activity could be blocked by heat inactivation (see table 1). Future studies will have to identify which specific HDM components are responsible for the effects on epithelial barrier function in relation to allergic sensitisation.
We have previously published that CCL17 is produced by epithelial cells through epidermal growth factor receptor-dependent signalling upon downregulation of E-cadherin.8 We report here that the extract that most profoundly disturbed epithelial barrier function also uniquely increased CCL17 levels in the mouse lungs. In line with our data, this specific extract has recently been shown to increase epidermal growth factor receptor activation in mice, contributing to AHR34 and mucus production/goblet cell hyperplasia.35
In summary, we demonstrate that allergic sensitisation to HDM does not critically involve serine/cysteine protease activity, but is related to the disruption of epithelial barrier function and pro-inflammatory epithelial responses. Our data demonstrate that HDM can induce delocalisation of E-cadherin and TJ proteins independently of serine/cysteine proteases, possibly by heat-insensitive proteases, yet the exact mechanism has to be established. It is important to further unravel these mechanisms, since the extract with the most detrimental effects on barrier function in vitro also showed increased IL-5 and CCL17 levels and induced allergic sensitisation, eosinophilic airway inflammation, AHR and goblet cell hyperplasia in vivo. Based on our data, we propose that epithelial barrier function serves as an important target for future therapeutic strategies in asthma.
Acknowledgments
We thank Citeq Biologics for the kind gift of their extract and L. den Boef (Laboratory of Allergology and Pulmonary Diseases, Department of Pathology and Medical Biology, University Medical Center Groningen, Groningen, The Netherlands) for technical assistance with the Flexivent experiment. The authors also thank Professor Dr D S Postma for valuable discussions.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
Footnotes
S Post and M C Nawijn contributed equally to the manuscript.
A J M van Oosterhout and I H Heijink share senior authorship.
Funding This study was supported by a grant from the Netherlands Asthma Foundation (NAF 3.2.07.019).
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.