Article Text
Abstract
Background The origins of respiratory disease might be traced back to exposures during fetal life. The aim of the present study was to explore whether there was a relationship between fetal size and respiratory outcomes at 5 years of age in the context of fetal exposure to vitamin E.
Methods A longitudinal birth cohort study was recruited (n=1924). Antenatal ultrasound scan results were identified and the following recorded: crown–rump length (CRL) in the first trimester; femur length (FL) and biparietal diameter (BPD) in the second trimester. Maternal plasma α-tocopherol (vitamin E) was measured at the time of the first trimester scan. At 5 years, wheeze and asthma symptoms were reported by questionnaire, and spirometry was measured.
Results CRL, spirometry and questionnaire data at 5 years were available for 835, 579 and 1145 individuals, respectively. There were positive associations between CRL and forced expiratory volume in 1 s (FEV1; 5 ml increase in FEV1 per mm CRL, p=0.001, n=283), forced vital capacity (FVC; 6 ml increase in FVC per mm CRL, p=0.001) and forced expiratory flow between 25% and 75% of FVC (FEF25–75; 0.008 ml/s increase in FEF25–75 per mm CRL, p=0.023), and inverse relationships with CRL and current wheeze (OR 0.59 per CRL quartile, p=0.026, n=547) and asthma (OR 0.55 per CRL quartile p=0.011). CRL was positively associated with maternal plasma α-tocopherol (p=0.002).
Conclusions These findings support the concept of very early fetal programming of respiratory disease. Maternal vitamin E status may be one determinant for growth of the fetus and fetal lungs during early pregnancy.
- Asthma mechanisms
- paediatric asthma
Statistics from Altmetric.com
Introduction
The potential for adult disease to be determined by influences active during fetal life is termed developmental programming.1 The concept of developmental programming was first proposed by Barker2 who suggested that ‘fetal undernutrition in middle to late gestation, leads to disproportionate fetal growth and programs later [coronary heart] disease’. The Barker hypothesis is one of a number of scenarios where fetal influences might impact on postnatal disease.
Asthma is a common condition characterised by abnormalities in lung function that are established shortly after birth and track into later life3 4; thus developmental programming is likely to be relevant to asthma. Evidence that events occurring in fetal life may be relevant to the respiratory system is provided in studies where birth weight is positively related to lung function in adulthood.5 6 The timing of in utero influences on the developing respiratory system is unclear. The Dutch famine study7 reported increased respiratory symptoms, but not abnormal lung function, among adults whose mothers were malnourished during the mid-trimester. We have reported associations between reduced maternal vitamin E intake during the last trimester and asthma symptoms, and between reduced maternal plasma α-tocopherol in the first trimester and reduced postbronchodilator forced expiratory volume in 1 s (FEV1) in 5-year-old children8; we have demonstrated positive correlations between plasma α-tocopherol and vitamin E intake in this cohort.9 The results from these studies7 8 suggest that exposures from as early as the first trimester may be important determinants of lung development and respiratory disease during childhood. Other groups have reported associations between maternal vitamin E intake and fetal growth,10 11 indicating that an association between vitamin E and respiratory outcomes may be mediated by fetal size and/or growth.
The age at onset of the association between abnormal lung function and asthma symptoms, and the nature of relevant exposures, might be determined in a study where measurements of fetal well-being during the first and second trimesters were related to postnatal lung function and respiratory outcomes. Here, we describe outcomes in such a study. We test two hypotheses: first that reduced fetal size in the first and second trimester is associated with reduced lung function and increased asthma symptoms at age 5 years; and secondly that the association we have previously reported between maternal vitamin E status during pregnancy and childhood respiratory outcomes is, in part, mediated through effects on fetal size.
Methods
Study design
Study subjects were participants in a birth cohort study designed to explore the relationship between antenatal dietary exposures and asthma outcomes in childhood.12 Mothers were recruited at a routine first trimester ultrasound scan to date the pregnancy; the median gestational age was 11 (IQR 8–12) weeks. A history of maternal smoking and asthma was noted and blood was taken for (non-fasting) maternal plasma vitamin E (α-tocopherol) determined by normal phase high-pressure liquid chromatography and adjusted for cholesterol. In the second trimester (median 20 weeks gestation, IQR 20–20), fetal measurements were recorded at a detailed anomaly scan. At 32 weeks gestation, maternal intake of vitamin E was determined by a food frequency questionnaire.11 Birth weight, length and head circumference were recorded. When the child was aged 5 years, a modified ISAAC (International Study of Asthma and Allergies in Childhood) questionnaire13 was completed by parents and returned by post. Height and weight, spirometry and skin prick reactivity were determined in a representative subset. This study was approved by The North of Scotland Research Ethics Committees. Informed and written parental consent and verbal assent from children was obtained.
Antenatal scan measurements
These were collected retrospectively in 2007. Gestation was ascertained by either date of last menstrual period (LMP) for mothers with a regular 28-day menstrual cycle or crown–rump length (CRL) at booking scan. Gestation predicted by CRL was adopted in cases where the gestation from LMP and CRL differed by >7 days. Scans in the first and second trimester included assessments completed between 8 and 12 weeks or 18 and 22 weeks gestation, respectively. The CRL was measured at the first trimester scan, and femur length (FL) and biparietal diameter (BPD, inner–outer) were measured at the second trimester scan. Scans took place between July 1997 and October 1999. An ATL (Ultramark 4A) or Toshiba (SSA-240A or SSA-340A) ultrasound scanner was used to determine fetal measurements and calibrated in accordance with the manufacturer's recommendations. Measurements of the fetus and newborn were expressed as raw data and z-scores using the published equations (see online supplement). The interobserver coefficient of variation for CRL measurements is between 8% and 10%.14
Spirometry and skin prick testing
Spirometry was measured using a pneumotachograph with incentive display software (Spirotrac IV version 4.22, Vitalograph, Bucks, UK). Spirometric variables were expressed as raw data and z-scores.15 The skin prick test was used to determine reactivity to cat fur, timothy grass, egg and house dust mite (allergens supplied by ALK, Hungerford, UK). The negative control was 0.9% saline and the positive control was histamine 10 mg/ml. A positive response was defined as a weal of ≥3 mm in its longest dimension and atopy was defined as at least one positive response.
Statistical analysis
The cohort was first analysed after excluding children of low birth weight (LBW; ie, <2.5 kg) because of a risk that the LBW group would skew the overall results due to an association between LBW for gestation and infant lung function.16 Analyses were then repeated including the whole cohort. Linear regression models were created to relate spirometric indices (outcome variables; ie, forced vital capacity (FVC), forced expiratory flow between 25% and 75% of FVC (FEF25–75), and prebronchodilator and postbronchodilator FEV1) to fetal measurement (predictive variable; ie, CRL, FL and BPD) whilst adjusting for these factors: gestational age at scan, sex, maternal smoking status at first trimester scan, history of maternal asthma, maternal height, Scottish Index of Multiple Deprivation (SIMD),17 birth weight and child's height aged 5 years. Binomial logistic regression models were used to study the odds for respiratory symptoms as a function of increasing fetal measurements also adjusting for factors included in the linear regression models (excluding maternal height) and additionally birth order, breast feeding, use of antibiotics by the child in the first year of life and maternal intake of vitamin C,11 vitamin D18 and zinc.11 Individuals were dichotomised about the median CRL and BPD z-score values and placed into one of these four resulting groups: persistent high growth (high CRL and BPD); growth deceleration (high CRL and low BPD); growth acceleration (low CRL and high BPD): and persistent low growth (low CRL and BPD). Differences in lung function and respiratory outcomes between these groups were described using multivariate linear and logistic regression models, respectively, with adjustment for confounders as previously described. To investigate whether the associations between maternal vitamin E status during pregnancy and childhood outcomes8 could be modified through effects on fetal growth, the previously described multivariate linear and logistic analyses8 were repeated including maternal vitamin E status (plasma α-tocopherol or vitamin E intake) SPSS (SPSS, version 15.0.0) software was used and significance was assumed at 5%.
Results
Study subjects
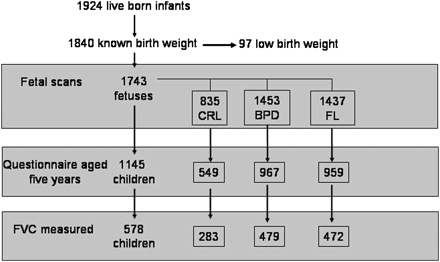
Figure 1 displays the number of individuals in whom data were available. There were 2000 pregnant women recruited of whom 1924 had live-born singleton infants and, of these, 1840 were born at Aberdeen Maternity Hospital, including 97 of LBW (<2.5 kg, of whom 14 had gestation <35 weeks). The following were recorded: CRL in 903; BPD in 1560 (including 806 where CRL was also measured); FL in 1544; questionnaire data at 5 years of age in 1187; skin prick reactivity in 689 (175 positive); and reliable spirometry in 608. Table 1 demonstrates that the 283 children where measurements of CRL and spirometry were available were representative of the whole cohort, with the exception of maternal smoking during pregnancy. We have shown that mothers who smoked were less likely to respond at 5 years.8
CONSORTdiagram demonstrating the number of individuals where fetal scan data could be matched to data at 5 years of age.
Comparison of outcomes between individuals where measurements of crown–rump length (CRL) at 10 weeks gestation and forced vital capacity (FVC) at 5 years of age were both available, and the whole cohort
Relationship between fetal measurements and spirometry
There were positive relationships between CRL and spirometric indices at age 5 years (table 2). There were also positive associations between BPD and postbronchodilator FEV1 and FVC (table 2). There were no associations between FL and spirometric indices.
Increases in spirometric indices at age 5 years per mm increase in fetal size
Relationship between fetal measurements, respiratory symptoms and atopy
For each millimetre increase in CRL in the first trimester scan, the odds of wheezing ever by 5 years of age fell by 4% (95% CI 1% to 7%; p=0.034) and the odds of having asthma ever by age 5 years fell by 5% (95% CI 1% to 9%; p=0.030) (table 3). The relationship between CRL and wheeze ever is presented in figure 2. Fetal measurements at 20 weeks were inversely associated with the risk for asthma ever and doctor-confirmed asthma (table 3). Associations between fetal measurements at the second trimester scan and asthma symptoms were similar when FL and BPD were expressed as categorical (ie, quartiles) and continuous variables. There was no relationship between any fetal measurement and childhood atopy.
ORs and 95% CI for asthma symptoms and per mm increase in fetal measurement
OR for wheeze ever at age 5 years in children groups by first trimester crown–rump length (CRL), p trend=0.028. Adjusted for gestational age at scan, sex, maternal smoking status at first trimester scan, history of maternal asthma, birth order, Scottish Index of Multiple Deprivation (SIMD), birth weight, breast feeding, use of antibiotics by the child in the first year of life and maternal intake of vitamin C, vitamin D and zinc. n indicates the total number of individuals included in the analysis.
Characteristics of individuals categorised by high or low CRL and BPD (growth trajectory)
There were 806 individuals in whom an assessment of fetal growth could be made in both the first (CRL) and second trimester (BPD). These were categorised as 302 with persistent high growth (high CRL and BPD), 103 with growth deceleration (high CRL and low BPD), 154 with accelerating growth (low CRL and high BPD) and 247 with persistent low growth (low CRL and BPD). Individuals with persistent low growth were at increased risk for wheeze in the past 12 months at the age of 5 years, wheeze ever, wheeze in the last 12 months, seeing the doctor for wheeze, asthma ever and doctor-confirmed asthma, and also had reduced FVC and postbronchodilator FEV1 compared with those with persistent high growth (table 4). The groups with accelerated or decelerated growth were not at altered risk for wheeze compared with the reference group. The group with growth acceleration had reduced prebronchodilator FEV1, postbronchodilator FEV1, FVC and FEF25–75 compared with the persistent high growth group (table 4). When FL replaced BPD as an index of second trimester fetal growth (supplementary table 1 online), the groups with persistent low growth and growth acceleration were at increased risk for wheeze ever, wheeze in the past 12 months and seeing the doctor for wheeze in the last 12 months, and the latter group also had reduced FVC compared with the reference group. Consistent associations between changing CRL and BPD and respiratory outcomes at age 5 years were also seen when males and females were considered separately (supplementary tables 2 and 3 online); the associations were of a greater order and higher significance in females compared with males. A potential interaction between maternal smoking status and growth trajectory group was explored using an interaction term between these variable where spirometric indices and respiratory symptoms were outcome variables; in none of the analyses was the interaction term significant.
Asthma risk factors, birth weights and asthma symptoms in groups categorised by high or low z-score for first trimester crown–rump length and second trimester biparietal diameter
Maternal plasma α-tocopherol, CRL and asthma outcomes
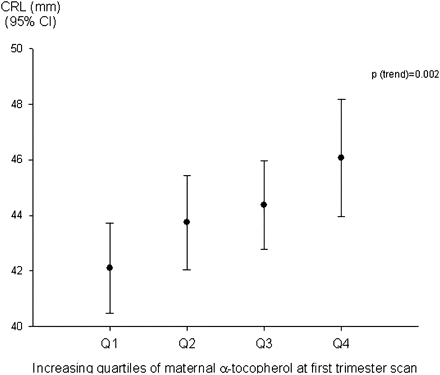
There was a positive association between maternal plasma α-tocopherol and CRL expressed as absolute measurement (p=0.002) (figure 3) and z-score (p=0.005). There was no relationship between maternal α-tocopherol and BPD or FL z-scores (respective p values 0.537 and 0.066). Associations between increased maternal plasma α-tocopherol/vitamin E intake and increased lung function and reduced asthma symptoms8 became statistically non-significant when absolute CRL was included in the models (table 5): similar associations were seen when CRL was expressed as z-score.
Mean crown–rump length (CRL) and maternal α-tocopherol at first trimester scan, p trend=0.002.
Odds for asthma outcomes in the context of maternal vitamin E exposure with and without consideration of first trimester fetal size (crown–rump length, CRL)
Relationship between birth weight, maternal vitamin E and asthma outcomes
These results are presented in the online supplement.
Analyses of the whole cohort (including LBW individuals)
These results are displayed in supplementary table 4. Inclusion of individuals of LBW increased the magnitude of the association between maternal vitamin E and respiratory symptoms and reduced the magnitude of that between CRL and respiratory symptoms. The relationship between spirometry and CRL was unchanged when all individuals were included in the analysis.
Discussion
We believe this to be the first study to explore whether antenatal measurements of human fetal size are related to postnatal respiratory outcomes, and we present two original findings. First, reduced fetal size in the first trimester was associated with reduced childhood lung function and increased asthma symptoms, independent of anthropometric measurements at birth and in childhood. Secondly, vitamin E appeared to be important to the relationship between first trimester fetal size and asthma outcomes. Our findings support the concept of developmental programming and suggest that the time at which airway development may be influenced by the environment begins in the first trimester and, in addition, implicate vitamin E as a growth factor important in lung development. The results presented here are based on a birth cohort where fetal measurements and outcomes at age 5 years were missing in a large proportion of individuals. For this reason, these findings need to be interpreted with caution and require confirmation elsewhere.
Humans pass more significant developmental stages before birth than afterwards, and the principle of developmental programming is highly plausible and the underlying mechanism is not clear. The initial ‘fetal origins’ hypothesis proposed fetal malnutrition as the mechanism for programming later morbidity19; a defect in early placentation may result in fetal growth failure and increased production of pregnancy-associated plasma protein A which is associated with coronary artery disease.20 Alternative hypotheses to explain developmental programming include fetal overexposure to corticosteroids21 and in utero insulin resistance.22 The present observational study therefore provides evidence in human subjects that an early fetal exposure (eg, vitamin E or associated nutrient) might be one factor which influences antenatal development, with implications for postnatal morbidity.
Other birth cohort studies have demonstrated ‘tracking’ in lung function from very early infancy into childhood4 and early adulthood,3 suggesting that reduced lung function is determined before birth.23 The present study extends these findings and demonstrates that childhood lung function may be determined in the first trimester. CRL incorporates thoracic length and it is reasonable to assume that CRL is closely related to lung volume, as length and height are in infants and children.
Fetal growth is influenced by many factors and, whilst it is recognised that severe maternal malnutrition in developing countries has an adverse affect on fetal growth,24 the present study suggests that suboptimal micronutrient intake by mothers in developed countries may have developmental and long-term health consequences for children. Previous studies have implicated vitamin E in fetal growth by demonstrating associations between α-tocopherol at 16 weeks and fetal growth at 28 weeks12 and also vitamin E intake in the second trimester and birth weight.10 The present study provides further evidence that vitamin E may be an important factor in fetal growth and also, for the first time, a growth factor for the developing respiratory system; this adds to the current understanding of early life influences on lung development. One of our group has recently hypothesised that maternal vitamin E intake during pregnancy influences the development of childhood asthma by affecting fetal airway development and the first critical interactions between the child's immune system and environmental allergens.25 The concept that vitamin E may enhance fetal lung growth is supported by work in animal models26 27 and now by the present study. The growth-enhancing effect of vitamin E does not appear to extend to the limbs since FL in the second trimester was not associated with either maternal vitamin E or childhood lung function. In addition to vitamin E, other factors are also likely to influence fetal lung development, including exposure to products of tobacco smoke28 and air pollutants.29 Genetic and epigenetic factors controlling expression of ADAM33 (a disintegrin and metalloprotease 33)30 and VEGF (vascular endothelial growth factor)31 may also be important to the control of in utero branching morphogenesis. Vitamin E has the potential to influence airway development because it is known to influence gene expression and27 and airway epithelial cell signalling,32 and has the potential to impact on epigenetic mechanisms.33
The results of the present study complement those from our group where maternal smoking beyond the first trimester was associated with reduced fetal size in the second and third trimester and reduced spirometry in childhood.34 First trimester, but not second trimester, fetal size was related to childhood lung function in this population and it is possible that in utero smoke exposure during the second trimester may have confounded the relationship between fetal size and spirometry reported in this manuscript. Together, observations from this cohort suggest that different exposures may be acting at different stages in antenatal development, with implications for respiratory health after birth.
We have previously reported associations between maternal vitamin E intake and cord blood mononuclear cell responses after stimulation by allergens,35 suggesting that vitamin E influences the first interactions between the immune system and allergens. The present study does not detract from this finding because the first and second trimester measurements were not associated with atopic sensitisation, in keeping with our working hypothesis that vitamin E influences airway and immune development. In this birth cohort we have also reported associations between childhood respiratory outcomes and maternal vitamin D, and zinc intakes; however, the currently reported associations between CRL, vitamin E and childhood outcomes were independent of these other nutrients,11 suggesting perhaps that these other nutrients may be influencing immune development.
The analysis was restricted to the fetal measurements routinely recorded in our institution. CRL was not measured in the second trimester, whilst FL and BPD were not measured in the first trimester, and we have only been able to infer altered fetal growth trajectories between the first and second trimesters by comparing the measurements available. However, there is no fetal measurement which can be accurately made in both the first and second trimester; CRL is not routinely measured beyond 13 weeks gestation due to increasing variability in relation to gestation after the first trimester,36 whilst fetal FL and BPD are too small to measure in the first trimester. Thoracic circumference can be ascertained in the second and third trimesters,37 but is not routinely measured in our institution, and we were not able to study the relationship between thoracic size in the second trimester and postnatal respiratory outcomes. Finally, fetal abdominal circumference was not measured in the third trimester where it is the single best parameter for assessing growth and in uterine growth retardation (IUGR) because of preserved head growth associated with IUGR; third trimester scans are not routine in our institution. Despite these limitations, the present study was nonetheless able to describe consistent associations between the fetal measurements available and respiratory outcomes.
There are some factors which should be considered when interpreting the present results. Although maternal α-tocopherol was measured at the same time as the first trimester scan, maternal α-tocopherol data were not available in the second trimester, preventing us from describing any ongoing association with fetal size and respiratory outcomes as the pregnancy progresses beyond the first trimester. Tobacco smoking is associated with reduced vitamin E intake, and maternal smoking during pregnancy is associated with fetal growth restriction and adverse respiratory outcomes in children.28 38 Although the associations reported in this study were adjusted for the effects of maternal smoking, the results presented are based on a representative subset of a larger cohort in which there was an excess drop-out of individuals exposed to products of tobacco smoking during pregnancy. The under-representation of mothers who smoked during pregnancy in this study makes it more likely that the observed associations are underestimated than overestimated; for instance, a larger proportion of wheezy children with smoking-associated in utero growth restriction and low maternal vitamin E intake would make the observed associations between first trimester measurements and childhood asthma/wheezing symptoms/lung function stronger. Finally, statistical associations, such as those reported here, may not necessarily reflect causation.
In summary, the level of lung function and predisposition to asthma symptoms may be determined in the first trimester, and maternal vitamin E status appears relevant to this association. The children participating in this study were relatively young and the relationships reported here might be modified as the children grow older, although we predict that the association between fetal size and lung function will persist since abnormalities in lung function are retained from childhood into later life. We believe that there is now sufficient evidence to undertake intervention studies whereby maternal diets during pregnancy are either supplemented or modified to optimise vitamin E intake to recommended levels of intake.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
Funding This study was funded by grants from Asthma UK and Tenovus Scotland neither of whom contributed to the study design, data analysis or preparation of this manuscript. The researchers are independent of Asthma UK and Tenovus Scotland.
Competing interests None.
Ethics approval This study was conducted with the approval of the North of Scotland Research Ethics Committee.
Provenance and peer review Not commissioned; externally peer reviewed.