Article Text
Abstract
BACKGROUND Nitric oxide (NO) is released by activated macrophages, neutrophils, and stimulated bronchial epithelial cells. Exhaled NO has been shown to be increased in patients with asthma and has been put forward as a marker of airways inflammation. However, we have found that exhaled NO is not raised in patients with cystic fibrosis, even during infective pulmonary exacerbation. One reason for this may be that excess airway secretions may prevent diffusion of gaseous NO into the airway lumen. We hypothesised that exhaled NO may not reflect total NO production in chronically suppurative airways and investigated nitrite as another marker of NO production.
METHODS Breath condensate nitrite concentration and exhaled NO levels were measured in 21 clinically stable patients with cystic fibrosis of mean age 26 years and mean FEV1 57% and 12 healthy normal volunteers of mean age 31 years. Breath condensate was collected with a validated method which excluded saliva and nasal air contamination and nitrite levels were measured using the Griess reaction. Exhaled NO was measured using a sensitive chemiluminescence analyser (LR2000) at an exhalation rate of 250 ml/s. Fourteen patients with cystic fibrosis had circulating plasma leucocyte levels and differential analysis performed on the day of breath collection.
RESULTS Nitrite levels were significantly higher in patients with cystic fibrosis than in normal subjects (median 1.93 μM compared with 0.33 μM). This correlated positively with circulating plasma leucocytes and neutrophils (r = 0.6). In contrast, exhaled NO values were not significantly different from the normal range (median 3.8 ppb vs 4.4 ppb). There was no correlation between breath condensate nitrite and lung function and between breath condensate nitrite and exhaled NO.
CONCLUSIONS Nitrite levels in breath condensate were raised in stable patients with cystic fibrosis in contrast to exhaled NO. This suggests that nitrite levels may be a more useful measure of NO production and possibly airways inflammation in suppurative airways and that exhaled NO may not reflect total NO production.
- cystic fibrosis
- breath condensate
- nitrite
- exhaled NO
- airways inflammation
Statistics from Altmetric.com
Nitric oxide (NO) is an ubiquitous free radical possessing diverse physiological functions. In the lungs it mediates inflammatory responses and modulates airways smooth muscle contractility, pulmonary perfusion, and immune response.1-4 NO levels have been found to be raised in the exhaled breath of asthmatic subjects,5 ,6 raising the possibility of a role in the pathophysiology of inflammatory airways diseases. The inducible nitric oxide synthase (iNOS) gene, one of three isoforms responsible for the production of NO, is expressed more highly in the airway epithelium of asthmatic subjects compared with normal controls.7Inducible NOS can be upregulated by proinflammatory cytokines (IL-1β, TNFα, and IFNγ) and the bacterial product lipopolysaccharide (LPS).7 NO is also important in host defence against infection. Systemic NO production in bacterial infection is increased both in human and animal studies8 ,9 and in vitro studies of phagocytic cells, and a variety of microbial targets have demonstrated cytokine inducible microbiostatic or microbicidal activity which is l-arginine dependent and inhibitable by competitive NOS inhibitors.10 Taken together, these data suggest that exhaled NO would be raised in inflammatory airway diseases other than asthma and, particularly, in diseases where chronic bacterial infection or colonisation dominates—for example, bronchiectasis and cystic fibrosis. However, the opposite has been shown in studies of exhaled NO in patients with cystic fibrosis even during infective pulmonary exacerbations.11-13 There are a number of possible explanations for this, one of them being that NO in the gaseous phase may be “trapped” by the viscous secretions in suppurative lung conditions and be effectively removed by its reaction with reactive oxygen species or with water and oxygen. NO is a highly reactive molecule with multiple oxidation states and does not persist as the NO• gaseous moiety.2 It can be reduced, oxidised, or complexed with other biomolecules depending on the microenvironment in which it is synthesised. In an aqueous environment, such as the air-fluid interface of the airways, NO generation may be indicated by the formation of stable end products of NO metabolism, nitrite (NO2 –) and nitrate (NO3 –).14 It is thus possible that in the airways of patients with cystic fibrosis gaseous NO could have been converted to nitrite and nitrate close to the site of production before it is able to move into the airway lumen and be detected as gaseous NO.
We have developed a non-invasive method of measuring nitrite production in the airways and have investigated whether the nitrite concentration in the airways of clinically stable cystic fibrosis patients is raised compared with normal subjects. We also analysed the relationship of breath condensate nitrite with circulating plasma leucocytes, exhaled NO, conventional lung function measurements, and the use of inhaled steroids.
Methods
SUBJECTS
Twenty one clinically stable patients with cystic fibrosis (13 men) with defined genotypes were recruited. Lung function was variable, with a mean (SD) forced expiratory volume in one second (FEV1) of 57 (24)% predicted (range 20–95). The mean age of the patients was 26.0 (9.3) years. Twelve healthy normal volunteers (six men) acted as controls, with a mean age of 31.4 (4.9) years. All subjects were non-smokers and had no intercurrent respiratory tract infection (for patients with cystic fibrosis this was defined as a worsening of the clinical condition requiring intravenous antibiotics accompanied by a fall of 400 ml or 20% of usual FEV1), active allergic rhinitis, or concomitant airways diseases.
All patients with cystic fibrosis had spirometric tests and bronchial reversibility to salbutamol. Patients with reversible airways obstruction (increase in FEV1 of more than 175 ml after 2.5 mg nebulised salbutamol) and those with clinical evidence of asthma were excluded. Within the cystic fibrosis group nine were on inhaled steroids in dose ranging from 200 μg to1500 μg beclomethasone per day which was started by their usual physician as anti-inflammatory treatment. Three patients with cystic fibrosis were on nebulised recombinant DNase.
All patients gave informed consent and the study was approved by the local ethics committee.
STUDY DESIGN
Breath condensate nitrite and exhaled NO were measured in all patients on two separate days. On all occasions the patients were clinically stable and FEV1 was not significantly different (<175 ml). Breath collections were made 3–5 hours after physiotherapy. Lung function was also measured on the same day as the measurements. Fourteen subjects consented to venepuncture for measurement of circulating white cell count and differential white cell count (neutrophils, eosinophils, monocytes, and lymphocytes). This was performed on the day of breath collection.
MEASUREMENT OF EXHALED NO
Exhaled NO was measured using a sensitive chemiluminescence analyser (LR2000, Logan Research Ltd, Kent, UK) with a detection limit of 0.1 ppb NO. The analyser was calibrated daily with NO/N2calibration gas containing 103 ppb NO (BOC, Guildford, UK). The subject inspired to total lung capacity (TLC) and, with no breath holding, exhaled gently into a sampling tube against a flow resistor. The subject exhaled at a constant rate to maintain a constant mouth pressure of 4–5 cm H2O by observing a visual display of this pressure. This method has been shown previously to exclude nasal passage air contamination of the sampled air, probably due to elevation of the soft palate against the oropharynx.15 All subjects maintained an exhalation flow rate of 250 ml/s. NO levels were taken from the plateau at the end of exhalation and the mean of triplicate measurements was used as the representative value.
OPTIMISATION OF BREATH CONDENSATE COLLECTION
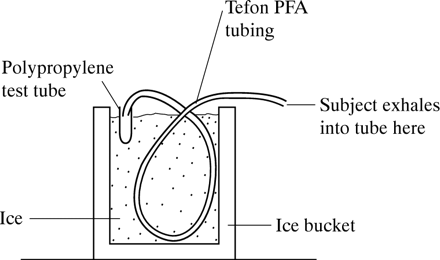
Breath condensate was collected using a novel method where the subject inspires repeatedly to TLC and exhales into 1.5 m Teflon perfluoroalkoxy (PFA) tubing with 0.5 cm internal diameter, immersed in ice (fig 1). This yielded 1 ml of breath condensate within five minutes. Nitrite was measured within 15 minutes of collection. Two factors may affect nitrite levels in breath condensate: (1) saliva contamination during condensate collection and (2) contribution of nitrite levels from nasal air, since nasal NO has been measured to be at least 50 times higher than lower respiratory tract NO.16 The method was thus tested for salivary and nasal air contamination. Subjects were instructed to maintain a dry mouth during the procedure by periodically swallowing their saliva. In 15 subjects with cystic fibrosis breath condensate samples collected over six minutes were each tested for salivary amylase levels using a reflectrometric dry slide method with a Vitros analyser (Ortho Clinical Diagnostics, Strasbourg). Two samples were “spiked” with saliva to ensure that this can be picked up with our method of detection. With regard to nasal air contamination, two subjects exhaled into the Teflon tube in the standard fashion while their noses were being flushed with 100% helium (BOC, Kent) delivered by nasal canulae. (The helium flushing started after inspiration). Expirate emerging from the collecting tube was continuously sampled for helium detection during and for 15 seconds after the end of exhalation. In order to establish that this system could detect helium in the expirate, the subjects inhaled to TLC while the nose was flushed with 100% helium and then exhaled into the collecting system where expirate was tested for helium as above. The nitrite concentration in the breath condensate was determined by a colorimetric assay based on the Griess reaction17 where triplicates of 100 μl of breath condensate were reacted with 25 μl of Griess reagent (0.1% naphthylethylene diamine dihydrochloride, 1% sulphanilamide, 3% H3PO4) and measured at absorbance of 570 nm with a microplate reader (MR 710, Dynatech). The intrasubject reproducibility on different days (3–8 days each) was measured in seven normal subjects.
Diagrammatic representation of breath condensate collection system.
STATISTICAL ANALYSES
Comparisons between the two groups were made using the Mann-Whitney rank sum test. The correlation between exhaled NO and nitrite levels and nitrite and lung function (FEV1) were measured using the Spearman rank sum test. For the reproducibility of nitrite assays and exhaled NO, Bartlett’s test was first applied to test for heterogeneity of variance between individuals and then ANOVA was applied to give a pooled standard deviation and 95% confidence interval.
Results
VALIDATION OF BREATH CONDENSATE COLLECTION AND NITRITE ASSAY
Griess reaction standard curve
The assay was able to detect levels as low as 0.5 μM and a standard curve from 0 to 10 μM consistently showed a linear relationship with r = 0.99.
Salivary amylase
In all 15 samples of saliva tested no amylase was detected using the method described, suggesting no contamination of breath condensate with saliva. Spiking the samples with less than 0.5 ml of saliva showed levels of more than 20 000 IU salivary amylase in two samples.
Nasal air contamination
In both subjects inhaling to TLC while the nose was flushed with 100% helium showed detectable helium in expired air within the first 15 seconds (2.8% and 1.5% in the two subjects). However, exhaled air tested when helium was delivered to the nose after the start of exhalation showed no helium in the expirate. These data indicate that the method of exhaling against the resistance of the tubing is sufficient to exclude contamination of the expirate by nasal air, presumably by elevating the soft palate and sealing off the posterior nasopharynx.
INTRASUBJECT VARIATION
Bartlett’s test showed no significant difference between the individuals tested (p = 0.91), thus ANOVA to the whole group could be applied. This gave a pooled SD of 0.5 μM and a 95% confidence interval for any measure of ±1.12 μM.
NITRITE AND EXHALED NO LEVELS IN CYSTIC FIBROSIS PATIENTS COMPARED WITH CONTROLS
We found nitrite levels to be significantly higher in patients with cystic fibrosis than in normal subjects (median 2.15 μM versus 0.36 μM, p<0.001; fig 2) and there was a positive correlation between circulating leucocytes and neutrophils (r = 0.6, p = 0.04 andr = 0.6, p = 0.03, respectively, Spearman’s rank order correlation; fig 3). In contrast, exhaled NO values were not significantly different compared with the normal range (median 3.8 (95% CI 3.4 to 5.2) ppb versus 4.4 (2.9 to 6.3) ppb). Nitrite levels were not significantly different in patients who were and were not receiving inhaled steroids (table 1). We found no correlation between breath condensate nitrite and lung function and no correlation between breath condensate nitrite and exhaled NO (p = 0.14 and p = 0.33, respectively, Spearman’s rank correlation test).
Distribution of nitrite measurements in study groups. Median levels in subjects with cystic fibrosis were significantly higher than normal controls regardless of usage of inhaled steroids.
Relationship between circulating plasma neutrophils and nitrite levels in breath condensate, both measured on the same day. Correlation tested with Spearman’s rank order correlation test (there are two overlapping points).
Nitrite levels in study groups and effect of inhaled steroids on nitrite levels in patients with cystic fibrosis
Discussion
NO production from the airways has been implicated as a possible marker of airways inflammation. Indeed, exhaled NO has even been put forward as a new lung function test.18 However, in reality many questions remain unanswered with regard to exhaled NO. There is no clear information as to the major cellular source of NO detected in the exhaled breath—does it originate principally from the inflamed epithelium or from inflammatory cells? The anatomical source of exhaled NO also remains unclear—it could arise from the central or peripheral airways or alveoli and there is no evidence of the relative contribution of each of these sites. Further, it is uncertain what proportion of total NO production is reflected by exhaled gaseous NO since a large proportion could theoretically have reacted close to its site of production. We have demonstrated that, compared with normal subjects, nitrite levels in breath condensate are higher in patients with cystic fibrosis but this was not the case with exhaled NO. This suggests that exhaled NO may not reflect total NO production in the airways, particularly if the airways are chronically suppurative. It is likely that NO, as it is being produced, is converted to nitrite and possibly nitrate (although this is not measured here) before it has the chance to diffuse into the lumen as gaseous NO. Excess secretions and mucus in the airways of patients with cystic fibrosis may inhibit diffusion of gaseous NO into the airways lumen and encourage interaction with the aqueous epithelial fluid lining, forming nitrite and nitrate. It is possible that increased levels of gaseous NO reflect very high levels of NO production which is surplus to that which has metabolised at the site of production. We have found that inhaling high concentrations of NO gas (80 ppb) and ambient NO levels as high as 100 ppb did not affect the exhaled NO (5 ppb).19 This suggests that, in order to detect increased levels of NO in the end expired gas, a very high level of NO has to be produced continuously since, in the time it takes for inhaled air to reach the lower airways and to be exhaled again, levels as high as 80 ppb have either degenerated, diffused into the pulmonary circulation, or metabolised to other products. If this is the case, it may be argued that no NO would be measured at all in normal subjects. We speculate that the levels seen in normal subjects represent background levels produced by the upper airways (trachea) and that lower airways production in normal uninflamed airways is probably not detectable as exhaled gaseous NO.
Breath condensate nitrite, like exhaled gaseous NO, gives no information as to the cellular source of NO production. A preliminary study by Meng and colleagues suggested that iNOS in cystic fibrosis epithelium (taken from lung explants of patients with cystic fibrosis) is absent although inflammatory cells around the cystic fibrosis epithelium continue to express iNOS normally.20 Our finding is not inconsistent with this since the nitrite levels that we measured may have reflected NO produced by inflammatory cells. This is supported by the relationship with circulating plasma neutrophils. Grasseman and colleagues have also reported high levels of a related NO metabolite, nitrate, in bronchoalveolar lavage fluid of children with pneumonia21 and, more recently, nitrite and nitrate levels in the sputum of patients with cystic fibrosis,22supporting the hypothesis that, in inflamed airways, a significant proportion of NO from the lower airways may have been degraded by oxidation to nitrite and nitrate. It is likely that NO generation is high in cystic fibrosis airways and is mainly attributable to inflammatory cell production, but that it is not detectable in exhaled air. Other factors may contribute to its removal from detection as gaseous NO—for example, reaction with other reactive oxygen species such as superoxide to form peroxynitrite.
The finding that nitrite did not correlate with FEV1suggests that it may reflect ongoing inflammation rather than the end result of airways damage. Again, this is supported by the correlation between expired nitrite and circulating total white cell count and neutrophils. We were surprised that inhaled steroids did not appear to affect nitrite levels but in these patients it is possible that large parts of the airways were inaccessible to inhaled medication. Compliance with medication in this group of patients is also questionable.
Grasseman and colleagues also showed that nitrite is raised in the saliva of patients with cystic fibrosis compared with normal controls.22 It may be argued that saliva could evaporate into the exhaled breath and may contain nitrite. While we could not positively exclude gaseous contribution of saliva derived nitrite in the breath condensate as we could for actual saliva in the condensate collection, we believe it unlikely that there would be any significant such contribution since the surface area of the airway epithelial lining fluid far exceeds that of the buccal cavity. Thus contribution of salivary condensate to lower respiratory tract condensate would be minimal.
We have used nitrite rather than nitrate because of our experience with this assay. Recent data have suggested that nitrite may be the main stable end product of NO metabolism and the redox reactions of NO species with oxygen suggest that both nitrite and nitrate would be formed in equal quantities.23 Furthermore, we have previously found that nitrate and nitrite generation in cultured chondrocytes correlated strongly with each (r= 0.99).24
In summary, we have shown that, with our method, breath condensate nitrite is an easy and non-invasive assay which avoids the main confounding effects of nasal air and saliva contamination. It is raised in clinically stable patients with cystic fibrosis compared with normal controls and is possibly a more sensitive marker of airways NO production than exhaled NO. It may be a more useful marker of airways inflammation than exhaled gaseous NO in suppurative airways disease.
Acknowledgments
The authors would like to thank Dr Peter Teague (Statistician, MRC Human Genetics Unit, Edinburgh) for statistical advice and Miss Joanne Samways (Respiratory Unit, Western General Hospital NHS Trust, Edinburgh) for assistance with setting up the helium analyser. Dr L P Ho and the study were supported by the MRC programme grant No. G9313618.