Article Text
Abstract
Background Assessing functional impairment, therapeutic response and disease progression in patients with idiopathic pulmonary fibrosis (IPF) continues to be challenging. Hyperpolarized 129Xe MRI can address this gap through its unique capability to image gas transfer three-dimensionally from airspaces to interstitial barrier tissues to red blood cells (RBCs). This must be validated by testing the degree to which it correlates with pulmonary function tests (PFTs) and CT scores, and its spatial distribution reflects known physiology and patterns of disease.
Methods 13 healthy individuals (33.6±15.7 years) and 12 patients with IPF (66.0±6.4 years) underwent 129Xe MRI to generate three-dimensional quantitative maps depicting the 129Xe ventilation distribution, its uptake in interstitial barrier tissues and its transfer to RBCs. For each map, mean values were correlated with PFTs and CT fibrosis scores, and their patterns were tested for the ability to depict functional gravitational gradients in healthy lung and to detect the known basal and peripheral predominance of disease in IPF.
Results 129Xe MRI depicted functional impairment in patients with IPF, whose mean barrier uptake increased by 188% compared with the healthy reference population. 129Xe MRI metrics correlated poorly and insignificantly with CT fibrosis scores but strongly with PFTs. Barrier uptake and RBC transfer both correlated significantly with diffusing capacity of the lungs for carbon monoxide (r=−0.75, p<0.01 and r=0.72, p<0.01), while their ratio (RBC/barrier) correlated most strongly (r=0.94, p<0.01). RBC transfer exhibited significant anterior-posterior gravitational gradients in healthy volunteers, but not in IPF, where it was significantly impaired in the basal (p=0.02) and subpleural (p<0.01) lung.
Conclusions Hyperpolarized129Xe MRI is a rapid and well-tolerated exam that provides region-specific quantification of interstitial barrier thickness and RBC transfer efficiency. With further development, it could become a robust tool for measuring disease progression and therapeutic response in patients with IPF, sensitively and non-invasively.
- idiopathic pulmonary fibrosis
- imaging/CT MRI etc
- interstitial fibrosis
Statistics from Altmetric.com
Key messages
What is the key question?
How can we directly and sensitively measure the regional functional impairment caused by idiopathic pulmonary fibrosis (IPF)?
What is the bottom line?
Hyperpolarised 129Xe MRI provides non-invasive measures of pulmonary gas transfer at the alveolar capillary level that correlate robustly with diffusing capacity of the lungs for carbon monoxide, while revealing spatially resolved patterns of function that reflect a loss of gas transfer efficiency in the subpleural and basal lung in patients with IPF.
Why read on?
129Xe MRI demonstrated three different patterns of impaired gas exchange that could represent different disease stages or phenotypes, which may aid in understanding the significant heterogeneity in disease courses often seen in IPF.
Introduction
Idiopathic pulmonary fibrosis (IPF), the most common interstitial pneumonia, is a devastating disease with a poor prognosis and estimated survival of 2–3 years after diagnosis.1–3 Historically, treatment has been limited to lung transplantation,1 4 but today, patients are routinely treated with antifibrotic therapy using either the tyrosine kinase inhibitor, nintedanib, or the tumour growth factor-beta inhibitor, pirfenidone.1 5 6
However, treatment of IPF and development of new therapies continue to be hampered by the lack of reliable metrics to assess therapeutic response and disease progression. To date, the primary indicators have been forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO).1 While DLCO measures gas exchange, it has been difficult to standardise in multi-institutional trials.7 Thus, FVC is used as a surrogate endpoint in most clinical trials, despite the continued lack of consensus on a threshold to define clinically significant decline, predict outcomes and validate treatment decisions.1 8–10
The pathogenesis of IPF is thought to involve excess collagen deposition that limits the capacity of alveoli to participate in gas exchange.4 However, the disease is heterogeneous in its distribution, and this cannot be captured using global metrics. Thus, imaging is needed to highlight and quantify disease activity regionally. To this end, high-resolution CT has become a mainstay in IPF, detecting fibrotic changes and structural abnormalities to establish the presence of a usual interstitial pneumonia (UIP) pattern without the need for surgical lung biopsies.1 This has led to further interest in using quantitative CT to measure longitudinal change, assess disease severity and predict mortality.11–13 However, the structural alterations detected on CT often do not correlate well with patient symptoms.14
A promising modality by which to assess regional function is MRI using inhaled hyperpolarized (HP) 129Xe gas. This method is rapid, well tolerated15 and allows for gas uptake and transfer to be assessed in a spatially resolved manner. On inhalation, xenon diffuses from the alveoli, through pulmonary barrier tissues comprised of alveolar epithelial cells, interstitial tissues, capillary endothelial cells, and plasma, and finally into capillary red blood cells (RBCs). Moreover, in each of these compartments, 129Xe exhibits distinct resonance frequency shifts16 17 (figure 1A,B) that enable its uptake in them to be measured separately.18–22
(A) Diagram of gas-phase 129Xe transfer from alveoli to interstitial barrier tissues and capillary RBCs that comprise the dissolved phased signal. (B) The 129Xe spectrum exhibits three resonances in lung at 0 ppm, 198 ppm and 217 ppm corresponding to 129Xe in airspaces, dissolved in barrier and RBCs.
Previously, whole-lung spectroscopic measurements demonstrated that the ratio of 129Xe uptake in RBCs versus barrier was dramatically reduced in subjects with IPF compared with healthy volunteers.17 This work was subsequently extended to produce isotropic three-dimensional (3D) images of 129Xe in the airspaces, barrier and RBCs and qualitatively revealed that RBC signal was diminished or absent in regions where fibrosis was evident on CT.23 We have since developed methods that quantify 129Xe transfer MRI more rigorously to reveal preliminary evidence that in IPF 129Xe uptake in the barrier tissues is also significantly enhanced.24
In this work we sought to determine the correlation between biomarkers derived from quantitative 129Xe gas transfer MRI and established metrics and to characterise the spatial distribution of 129Xe gas transfer impairment. We hypothesised that 129Xe biomarkers would correlate more strongly with the pulmonary function test (PFT) measurements, FVC and DLCO, than with CT fibrosis scores.25 Furthermore, we hypothesised that the spatial distribution of 129Xe gas transfer MRI would follow known histopathological patterns in IPF. Specifically, we tested the hypothesis that enhancement of barrier uptake and impairment of RBC transfer would predominate in the basal and subpleural lung.1 26 27
Methods
Subject recruitment
This study was approved by the Duke Institutional Review Board. Written, informed consent was obtained from each subject prior to protocol recruitment. Data were acquired from 13 healthy volunteers (33.6±15.7 years, 9 men, 4 women) and 12 subjects with IPF (66.0±6.4 years, 10 men, 2 women). All subjects were at least 18 years old and had no history of cardiac arrhythmias. Healthy volunteers had no smoking history or diagnosed pulmonary conditions. All subjects underwent baseline pulmonary function testing to obtain FVC by spirometry and DLCO by the single-breath method. The diagnosis of IPF was determined using ATS criteria confirming a UIP pattern on CT or from surgical lung biopsy.1 All subjects with IPF had prior CT scans, with an interval of 5.1±4.9 months from their MRI scan. CT scans were evaluated by a chest radiologist using established criteria to produce a mean fibrosis score ranging from 0% to 100%.25 These scores were used to classify IPF disease severity as mild (<11%), moderate (11%–30%) or severe (>30%).25
129Xe polarisation and delivery
Isotopically enriched 129Xe gas (85%) was polarised to 20% (Model 2881, Polarean, Durham, North Carolina, USA) and dispensed into a Tedlar dose delivery bag as previously described.28 Subjects received a small dose (51 mL dose equivalent of pure HP 129Xe) for calibration and a larger dose (85 mL dose equivalent) for the imaging scan.29 These doses required xenon volumes ranging from 300 to 750 mL, which were expanded with high-purity helium to achieve a net 1 L volume, which subjects inhaled from functional residual capacity. They then held their breath for 13–15 s during scanning, while heart rate and oxygen saturation were monitored with an MR-compatible monitoring system (Expression Model 865214, Invivo, Orlando, Florida, USA).
Image acquisition and reconstruction
129Xe gas transfer MRI was acquired on a 1.5T scanner (GE EXCITE 15M4), using an interleaved radial acquisition of gas-phase and dissolved-phase images during a 15 s breath-hold.30 Images were acquired at an echo time (TE90≈ 0.9 ms) that allows the barrier and RBC signals to be separated by the 1-point Dixon method.23 This generated separate isotropic 3D images of the gas, barrier and RBC compartments with 6.3 mm isotropic voxels.
Image processing and analysis
The gas-phase images were rendered into quantitative maps by rescaling by their top percentile of intensities and assigning each voxel into one of six colour clusters. These clusters each span an intensity range equal to one SD of the ventilation reference distribution derived from a healthy cohort.31 The barrier and RBC images were divided on a voxel-by-voxel basis by the gas-phase intensities to create maps of barrier uptake and RBC transfer. These maps were also cast into colour clusters, each spanning one SD of the associated healthy cohort reference distribution as recently detailed.24 The resulting barrier maps reflect the amount of 129Xe uptake in interstitial tissues compared with healthy subjects. Similarly, the RBC transfer maps depict the amount of 129Xe that has reached the capillary RBCs, again relative to a healthy cohort. Each map was quantified by its mean, as well as by the percentage of voxels in the highest and lowest two clusters of their distribution. For barrier maps, the highest two clusters (BarrierHigh) contained the percentage of voxels falling three or more SD above the healthy reference mean. This metric thus reflected interstitial thickening. For the RBC maps, the lowest two clusters (RBCLow) contained the percentage of voxels falling more than one SD below the reference mean. This metric thus quantified the fraction of lung exhibiting poor gas transfer to RBCs.
Functional gradient analysis
To test the validity of the spatial distribution in functional gas transfer MRI, signal gradients were quantified in three directions. First, the lungs were divided into two equal volumes in the anterior/posterior direction to test the hypothesis that gravitational effects that increase tissue density and perfusion to the dependent lung in healthy subjects would be eliminated in IPF. Similarly, gradients were calculated in the apical/basal direction to assess the hypothesis that impaired function in IPF would show a basal predominance,26 and finally gradients in the central/peripheral direction were calculated to test the hypothesis that impaired function would also follow a peripheral predominance.27 This was done by defining the peripheral lung as the outermost 2–3 cm of the subpleural lung perimeter. All spatial gradients were calculated by dividing the mean signal difference between regions by their average.
Statistical methods
All statistical analyses were performed using JMP 12 (SAS Institute, Cary, North Carolina, USA). The unpaired two-tailed Student’s t-test was used to evaluate differences in the spatial distribution of gas transfer. Linear regression analysis and the Pearson correlation coefficient (r) were used to assess correlation between functional MRI metrics and CT fibrosis scores, as well as PFT results. For all comparisons, the level of significance was 5% (p<0.05).
Results
Single-breath, isotropic images of gas and dissolved-phase 129Xe were successfully acquired in all subjects and reliably decomposed into separate ventilation, barrier uptake and RBC transfer maps. Subject demographics, PFT results and functional imaging-derived ratios are summarised in table 1.
Subject demographics, PFT metrics and imaging-derived parameters
Representative gas transfer maps in healthy subjects versus subjects with IPF
Representative ventilation, gas transfer maps and CT images are depicted in figure 2A for a healthy control subject (FVC=97% predicted, DLCO=113% predicted). All maps consist largely of green voxels that represent ratios falling within ±1 standard deviation of the reference healthy mean. While all maps exhibited mean values close to the reference mean (ventilation=93%, barrier=120%, RBC=121%), they do exhibit notable gradients as both barrier uptake and RBC transfer increase towards the gravitationally dependent lung. However, only a modest percentage of voxels fell into BarrierHigh (0.5%) and RBCLow (10%). Low RBC transfer was seen primarily in the anterior, non-gravitationally dependent lung. This pattern of few pixels falling in BarrierHigh and RBCLow was generally found to exist in the healthy cohort as shown in table 1.
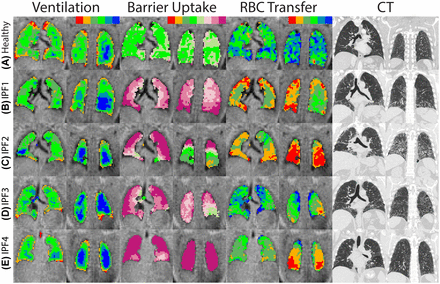
Central and posterior coronal slices of ventilation (red=ventilation defects, blue=high), barrier uptake (red=low, plum/orchid=high) and RBC transfer (red=low, blue=high), binning maps and CT images in one healthy subject and four subjects with IPF. (A) Healthy subject with FVC=97%, DLCO=113%, BarrierHigh=0.5%, RBCLow=10%. (B) IPF subject 1 with FVC=55%, DLCO=31%, BarrierHigh=74%, RBCLow=52%, CT fibrosis score=25.4%. (C) IPF subject 2 with FVC=42%, DLCO=23%, BarrierHigh=65%, RBCLow=65%, CT fibrosis score=34.2%. (D) IPF subject 3 with FVC=62%, DLCO=47%, BarrierHigh=61%, RBCLow=18%, CT fibrosis score=46.7%. (E) IPF subject 4 with FVC=77%, DLCO=54%, BarrierHigh=94%, RBCLow=36%, CT fibrosis score=10.4%. DLCO, diffusing capacity of the lungs for carbon monoxide; FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis; RBC, red blood cell.
Figure 2B depicts 129Xe functional maps in a subject with moderate IPF (FVC=55% predicted, DLCO=31% predicted, CT fibrosis score=25.4%). Although ventilation (mean=91%) was not significantly different from the healthy volunteer, the barrier signal was strikingly enhanced throughout the lung (206% of reference), with 74% of voxels falling in BarrierHigh. Enhanced barrier uptake was most notable in the subpleural regions, which was accompanied by reduced RBC transfer (57% of reference), with 52% falling into RBCLow. The corresponding CT images depict predominantly basal and peripheral reticulation and areas of ground glass attenuation, with mild traction bronchiectasis in the affected areas.
Figure 2C depicts ventilation and gas transfer maps in a patient with severe IPF (FVC=42% predicted, DLCO=23% predicted, CT fibrosis score=34.2%). Ventilation maps have a reduced mean (83% of reference), but the most striking feature is the highly elevated barrier uptake throughout most of the lung (200% of reference, BarrierHigh=65%). Similarly, this subject exhibited significant regions of low RBC transfer (49% of reference and RBCLow=65%). Low RBC transfer was most evident in the posterior and basilar lung. These matched well with extensive distortion of the lung architecture and honeycombing seen on CT. Interestingly, in portions of these posterior slices, the barrier uptake was in the normal range.
Figure 2D shows ventilation and gas transfer maps in another IPF subject with severe disease (FVC=62% predicted, DLCO=47% predicted, CT fibrosis score=46.7%). Here, ventilation is preserved (104% of reference), but barrier maps again exhibit significant enhancement throughout the lung (196% of reference, BarrierHigh=61%). However, 129Xe transfer to RBCs remained in the normal range through most of the lung, with a mean that exceeded the reference value (114%). Only a few regions of low RBC transfer were evident in the peripheral and posterior lung (RBCLow=18%). This patient had a CT scan notable for honeycombing and parenchymal changes consistent with a UIP pattern.
Figure 2E shows an IPF subject with mild disease (CT fibrosis score=10.4%) and the highest FVC (77% predicted) and DLCO (54% predicted) in the cohort. Again, ventilation maps resemble those of healthy subjects (104% of reference). However, this subject also exhibited the highest barrier uptake in the cohort (246% of reference, BarrierHigh=94%). The RBC maps depict normal gas transfer in the midlung but otherwise low RBC transfer in the apical and basal lung (mean=73%, RBCLow=36%.) In this patient with biopsy-confirmed UIP, CT showed a possible UIP pattern with a peripheral reticular pattern and mild traction bronchiectasis but no honeycombing.
Correlation with CT fibrosis scores
In most subjects with IPF, the spatial correlation between functional 129Xe transfer metrics and CT was not immediately evident. CT fibrosis score was weakly and insignificantly correlated with all MRI metrics: ventilation (r=0.09, p=0.77), barrier uptake (r=0.27, p=0.38), RBC transfer (r=0.21, p=0.48) and RBC/barrier ratio (r=−0.05, p=0.88).
Correlation with pulmonary function tests
Almost all image-derived average gas transfer metrics, ventilation, barrier uptake, RBC transfer and RBC/barrier ratio, correlated significantly with FVC and DLCO (figure 3); the only exception was between ventilation and DLCO. Ventilation correlated moderately and positively with FVC (r=0.39, p=0.04) but not significantly with DLCO (r=0.35, p=0.06). Barrier uptake correlated negatively and significantly with both FVC (r=−0.61, p<0.01) and DLCO (r=−0.75, p<0.01). RBC transfer correlated positively and significantly with both FVC (r=0.63, p<0.01) and DLCO (r=0.72, p<0.01). Overall, the strongest correlation was between RBC/barrier ratio and DLCO (r=0.94, p<0.01).
Mean 129Xe ventilation correlates only modestly with FVC and DLCO. However, barrier uptake, RBC transfer and RBC/barrier ratio all correlate well with FVC and DLCO. The correlation between RBC/barrier and DLCO (r=0.94) is particularly strong. In subjects with more than one MRI scan, correlation coefficients were calculated using all scans, while p values were derived using only the most recent time point to avoid assuming the independence of multiple measurements. DLCO, diffusing capacity of the lungs for carbon monoxide; FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis; RBC, red blood cell.
Spatial patterns of gas transfer
Although 129Xe gas transfer metrics did not correlate with structural CT scores, spatial gradients in 129Xe gas transfer followed expected patterns (figure 4). Ventilation maps in both cohorts demonstrated increasing intensity from the anterior to posterior and apical to basal directions but decreasing intensity from the central to peripheral direction. However, none of these gradients were significantly different between healthy subjects and subjects with IPF. Notably, barrier uptake exhibited a significantly stronger anterior to posterior gradient in healthy volunteers (22%) than subjects with IPF (8%, p<0.01). Yet, in the apical/basal or central/peripheral directions, barrier gradients were subtle (<10%) and were not significantly different between healthy subjects and subjects with IPF (p=0.63 and p=0.35, respectively). RBC transfer was characterised by a strong anterior to posterior gradient in healthy controls (mean=32%) that was eliminated in IPF (mean=2%, p<0.01). However, in IPF, RBC transfer exhibited a strong apical to basal gradient (−36%), significantly greater than in healthy volunteers (−12%, p=0.02). RBC transfer also exhibited a stronger central-peripheral gradient in IPF (−22%) than in healthy volunteers (−10%, p<0.01).
Ventilation, barrier uptake and RBC transfer gradients in three directions. In both healthy subjects and subjects with IPF, ventilation gradients follow similar spatial patterns. Barrier transfer exhibits a spatial gradient only in the anterior-posterior direction of gravity and only in healthy lungs. A similar but stronger gradient in RBC transfer was present in healthy subjects but absent in IPF. In IPF, RBC transfer follows a large apical/basal gradient and a moderate central/peripheral gradient, consistent with known patterns of disease. IPF, idiopathic pulmonary fibrosis; RBC, red blood cell. μ - average difference, H - healthy, *statistically significant.
Discussion
PFT correlations support functional imaging findings
The primary tools for diagnosing and monitoring IPF disease progression continue to be a combination of PFTs, CT scans and 6 min walk testing. Thus, the observation that ventilation MRI correlated only weakly with DLCO and insignificantly with FVC suggests that its regional assessment does not provide a meaningful measure of disease severity in IPF. However, it is noteworthy that 129Xe MRI-derived barrier uptake, RBC transfer and RBC/barrier ratio correlated well with DLCO, a direct marker of global gas exchange. In particular, RBC/barrier was exceptionally well-correlated (r=0.94), thereby conferring it with a strong physiological grounding. Unlike DLCO, however, RBC/barrier can be further dissected to identify changes in barrier and RBC transfer individually, as well as how these are distributed regionally. Moreover, because these metrics are calculated using signals localised within the airspaces, this inherently accounts for differences in the volume of inhaled xenon, making it potentially less effort dependent.32
Functional 129Xe MRI complements structural CT findings
The poor correlation between 129Xe MRI functional metrics and CT structural scores was particularly surprising. It is possible that these scores (which also correlated weakly and non-significantly with PFTs) reflect a limitation of the reader-based methodology. Recently, such reader methods were found inferior to computer-based CT analysis in predicting mortality in IPF,33 and one larger-scale study reported such more sophisticated densitometric and feature-based analysis to correlate positively with PFTs.34 However, a more fundamental explanation is that 129Xe MRI signal in barrier and RBC inherently arises only from the alveolar capillary unit and thus directly probes its function.35 Thus, one possible interpretation is that these direct regional gas exchange measurements are sensitive to disease at the alveolar level, which can differ from what might be inferred from large-scale structural findings on high-resolution CT.
The primary exception to the poor correlation between 129Xe MRI and CT was in late-stage IPF, where they appeared to agree visually, particularly in regions exhibiting extensive honeycombing. In such regions, both barrier uptake and RBC transfer were often diminished or absent. However, in less severe IPF, agreement was considerably weaker. In our cohort, we found numerous patients with definite UIP patterns on CT that did not demonstrate significantly impaired RBC transfer. In such cases, MRI paints a picture of preserved lung function despite extensive structural injury. On the other end of the spectrum, we also observed subjects where structural abnormalities were relatively few, but where MRI exhibited massive barrier enhancement throughout the lung, paired with regionally impaired RBC transfer. This may indicate significant disease activity that has not yet irreversibly remodelled the lung architecture.
Functional imaging gradients consistent with normal physiology and fibrosis distribution
It is encouraging that 129Xe MRI reveals spatial gradients that are consistent with both normal pulmonary physiology and the typical spatial patterns of disease in IPF. In a healthy subject lying supine, perfusion follows a gravitational gradient favouring the posterior lung.36 This was reflected in healthy volunteers exhibiting generally robust RBC transfer in the dependent lung, while any regions of low transfer were generally confined to the non-dependent, anterior slices. Similarly, barrier uptake also favoured the dependent lung in healthy subjects.
By contrast, such physiological gradients in 129Xe gas transfer were absent in IPF. Instead, barrier uptake was enhanced throughout the lung, consistent with a widespread pattern of fibrosis4 serving to override gravitational effects. Similarly, RBC transfer did not exhibit a gravitationally dependent pattern in IPF, possibly reflecting recruitment of gas exchange to anterior lung regions to maintain stable lung function.37 However, in the IPF cohort, several 129Xe spatial gradients emerged that were different from the healthy population. In particular, RBC transfer was significantly diminished in the basal and peripheral lung. This is where fibrosis and honeycombing are most commonly found on histology and CT.1 27
Models of gas transfer in IPF
129Xe MRI revealed four distinct patterns of gas exchange that often coexisted in the same patient (figure 5). The first is the essentially normal unit that predominates in healthy individuals (figure 5A), where inhaled 129Xe freely diffuses from the alveoli through the thin alveolar–capillary barrier to reach circulating RBCs. This results in barrier and RBC transfer being in the normal (green) range. Next is a region consistent with the classical model of diffusion limitation (figure 5B), where thickening of the alveolar–capillary membrane increases barrier uptake and delays transfer to capillary RBCs.17 Note that while xenon is classically considered to be a perfusion-limited gas, HP 129Xe MRI is inherently sensitive to diffusion impairment.17
Proposed models of gas transfer for various barrier uptake (red=low, plum/orchid=high) and RBC transfer patterns (red=low, blue=high). In a healthy lung (A), gaseous 129Xe efficiently diffuses from the alveolus, across a thin barrier to RBCs, resulting in signal intensities in the normal range for both compartments. In IPF, however, interstitial fibrosis thickens the barrier tissue, which increases xenon uptake. In some regions of barrier enhancement (B, arrows), diffusion across it is slowed, causing RBC transfer to decrease. As the disease progresses (C), scarring becomes so severe that 129Xe no longer diffuses into or through the barrier. Therefore, barrier uptake returns to the normal or low range, while RBC transfer is dramatically reduced. Most interesting are regions depicting the coexistence of increased barrier uptake with preserved RBC transfer (D, arrow). This may represent regions of disease activity that could be responsive to therapy.
Figure 5C depicts a pattern that is seen in subjects with end-stage disease, characterised by regions with seemingly normal or even low barrier uptake but almost no RBC transfer. When examined on CT, these regions reveal severe fibrosis and honeycombing. We suggest that such regions represent severe and permanent scarring that impairs 129Xe uptake into the barrier tissue, thereby almost entirely eliminating its subsequent transfer to RBCs. Over time, such distortion would likely also lead to absent perfusion.
Moreover, perhaps most interesting, is figure 5D, which illustrates a pattern of greatly enhanced barrier uptake coexisting with preserved RBC transfer. This is unexpected from conventional models in which a thicker barrier should impair gas transfer to RBCs. However, this finding agrees with histological patterns of IPF, where patchy fibrotic zones, honeycomb changes and fibroblastic foci are known to coexist with regions of normal lung.38 39 Given the relatively modest resolution of the images (250 mm3), a single voxel contains over 40 000 alveoli, many of which could plausibly be normal.40 Combined with the known histopathological temporal heterogeneity in IPF, this pattern may represent early disease where fibrotic changes are below the resolution of conventional CT. Such at-risk regions may be particularly valuable to monitor as IPF progresses and could help identify areas where novel therapies may elicit a positive response.
Study limitations and future directions
Although 129Xe MRI shows promise in delivering a wealth of regional functional information, it will be critical to apply these methods to larger cohorts and to establish short-term reproducibility. In the present study, we can only estimate an initial upper bound on variability by examining one healthy subject and seven subjects with IPF in the study who were scanned at two time points (average 7.7 months apart). This yielded within-subject coefficients of variation of ±16% for barrier uptake, ±18% for RBC transfer and ±11% for RBC/barrier. However, given that this sample includes several patients with IPF who exhibited significant clinical disease progression between scans, this crude estimate clearly represents only an upper bound. Perhaps a more representative benchmark is the whole-lung RBC/barrier spectroscopic ratio, which was previously shown to exhibit a same day variability of ±6.6%.17
One limitation of our study was the use of younger subjects in our control group. Although this provides a useful starting reference, the ageing lung is known to undergo physiological changes in chest wall compliance, respiratory muscle function and the lung parenchyma, all with the potential to affect gas exchange.41 42 Thus, future studies may benefit from including an older, age-matched population of healthy controls. Furthermore, all subjects inhaled 1 L of gas regardless of their lung volumes, which may affect our functional metrics. However, in our preliminary studies of healthy individuals scanned at both Functional Residual Capacity (FRC) + 1 L and after deliberate exhalation to FRC, gas transfer patterns were largely unchanged. Future studies would also benefit from determining and correcting for patient-specific haemoglobin levels.
We note several possible limitations that could explain the much stronger correlation of 129Xe MRI to PFTs. First, CT scoring was only available in the IPF cohort. However, even when eliminating healthy subjects from analysis, the 129Xe-PFT correlations remained robust for RBC/barrier with FVC (r=0.57, p=0.01) and DLCO (r=0.80, p=0.01). We also note that several subjects in the IPF cohort had CT and MRI scans several months apart. However, other studies of quantitative CT biomarkers in IPF detected changes in CT score over a 7-month period of 2%–3%.11 Thus, we expect the average 5-month separation of scans to be an unlikely explanation for the lack of correlation with 129Xe MRI.
Conclusions
These preliminary data suggest that 129Xe gas transfer MRI has the potential to add new insight by visualising functional change regionally. Because the method is non-invasive and avoids ionising radiation, it can be further developed to deliver robust and reproducible metrics to meet the pressing need for more sensitive markers of IPF disease activity. As a research tool, 129Xe MRI provides a fundamentally new way to characterise disease pathophysiology, which could translate to become clinically valuable in our efforts to understand the effects of emerging therapies and the mechanisms behind lung injury in IPF.
Acknowledgments
The authors wish to thank Sally Zimney for proofreading the manuscript.
References
Footnotes
Contributors CRR, PM and BD designed and implemented the study. JMW, SHR, ZW, MH, RSV, GMS and BD collected the data. JMW, SHR, ZW, MH and BD were responsible for data analysis. All authors contributed to the writing of the manuscript.
Funding Research reported in this publication was supported by the NIH/NHLBI R01HL105643 and R01HL126771, the Duke Center for In Vivo Microscopy, an NIH/NIBIB National Biomedical Technology Resource Center (P41 EB015897) and Gilead Sciences. LE received financial funding by the Swiss National Science Foundation.
Competing interests BD is founder and shareholder in Polarean Imaging, a company established to commercialise hyperpolarised 129Xe MRI technology.
Ethics approval This study was approved by the Duke Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.