Article Text
Abstract
Aims: The aims of the study were to compare bacterial recovery following storage of sputum samples at 20°C room temperature and 4°C (refrigerated) for 24 h, and to determine the effect of postage on viable bacterial numbers.
Methods: A total of 38 individual sputum samples from clinically stable patients with bronchiectasis were split into three equal aliquots and quantitative bacterial culture was performed (i) immediately, (ii) following storage at 4°C for 24 h or (iii) following storage at 20°C for 24 h. A further 42 sputum samples were split into two equal aliquots and quantitative bacterial culture was performed either immediately or following postage back to the laboratory by first-class mail from an outside location.
Results: The predominant organism could still be recovered following storage at 4°C and 20°C, but viable numbers were significantly reduced following storage at 4°C (p<0.004) by at least an order of magnitude (10-fold) in 24% of samples stored at 4°C compared with only 8% stored at 20°C. Posting samples back to the laboratory did not affect the recovery of bacterial species and there was no difference in viable numbers isolated.
Conclusions: The results suggest that storage at room temperature is preferable to refrigeration as it retains the species isolated and the viable number. The data also confirm that sputum samples can be posted to the laboratory from patients in the community without affecting qualitative or quantitative results.
Statistics from Altmetric.com
A range of bacterial species, most commonly Haemophilus influenzae, Streptococcus pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus and Moraxella catarrhalis, are frequently isolated in sputum from patients with chronic lung disease,1–3 and it is generally accepted that samples must be cultured as soon as possible after collection to obtain representative results.4 This is largely based on studies showing that the isolation rate of H influenzae and S pneumoniae is lower from “old” specimens compared with fresh samples.5 The reduced isolation rate has been attributed largely to the action of lysozomal enzymes in sputum and the inability of some bacteria to remain viable outside their normal environment.5 Sputum provides a good medium for growth of non-fastidious bacteria, and to maintain viability samples are often refrigerated prior to transport to the laboratory. P aeruginosa, however, still multiplies at or near refrigerator temperature (4°C), whereas H influenzae may be killed at low temperatures.6
There have been limited studies to investigate the effects of storage on the recovery of bacteria from sputum, with varying results observed. Storage at 4°C for 48 h has been reported to produce no detectable change in the numbers of S aureus, P aeruginosa, H influenzae or S pneumoniae recovered,7 whereas an earlier study of specimens kept at room temperature and transported by post showed a 50% decrease in the isolation rate of H influenzae and S pneumoniae compared with fresh samples from the same patients.5
The Working Party on the Retention and Storage of Pathological Records and Archives8 has recommended that all specimens should be retained for a period of 48 h following issue of final reports. Therefore, a prospective study was co-ordinated in three laboratories using individual standard operating procedures.9 Following storage of samples at 4°C for 48 h, identical results to those obtained initially were obtained in up to 75% of specimens, but H influenzae, M catarrhalis and S pneumoniae failed to be recovered in 8.7% of samples.9 That study did not, however, include samples from patients with bronchiectasis who are often colonised with several bacteria, and where overgrowth by organisms such as Pseudomonas spp. could be a significant problem.
Receiving samples from patients in the community without the need for specialised storage or transport conditions would be beneficial in the management and antibiotic treatment of patients with chronic lung disease, circumventing the need for open access clinics. Therefore the aim of this study was to compare viable bacterial numbers present in sputum stored for 24 h at 20°C and 4°C to determine any difference between numbers and species recovered when processed immediately and following storage. In addition, the effect on bacterial numbers of posting samples to the laboratory was investigated.
METHODS
Sputum samples
Samples were collected from clinically stable patients with radiologically proven bronchiectasis as part of routine management. The viable number of predominant bacterial species was determined using quantitative culture.10 Briefly, samples were homogenised with dithiothreitol and serially diluted with sterile saline. A 10 μl sample was spread onto chocolate, blood and MacConkey agars and plates were incubated at 36°C in 5% CO2 in air for 24–48 h. Plates yielding between 30 and 300 colonies were counted to determine viable bacterial numbers, expressed as colony forming units per millilitre (cfu/ml) of original sample. Individual species were identified using primary biochemical tests and final confirmation was performed using the API identification system (bioMérieux, Lyon, France).
Effect of storage on recovery and quantitation of bacteria
Thirty-eight samples were split into three equal aliquots by weight. Quantitative culture was performed immediately on the “fresh” aliquot, and the two remaining aliquots were stored at either 4°C or 20°C for 24 h prior to culture. Differences in numbers of the predominant organism recovered immediately after collection and following storage were compared.
Effect of posting on recovery and quantitation of bacteria
Forty-two samples were split into equal aliquots by weight. Quantitative culture was performed immediately on one aliquot and the remaining aliquot was posted by first-class post to arrive the following day. Quantitative culture was performed on the “posted” aliquot, and viable numbers compared to those obtained from the fresh aliquot.
Statistical analysis
Data were analysed for normality using Shapiro Wilk test. Since all data were normally distributed, paired t tests were used to determine the significance of any changes. A p value of <0.05 was used as conventional statistical significance.
RESULTS
Effect of storage on recovery and quantitation of bacteria
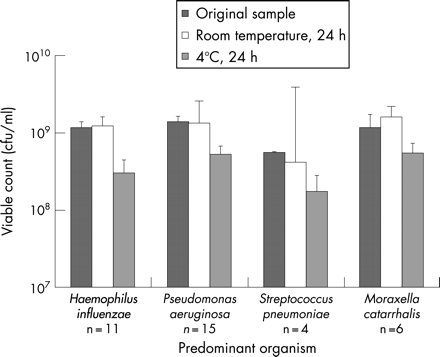
Quantitative culture of 38 samples immediately after collection and following 24 h at 4°C and 20°C showed varying results depending upon the predominant (organism with the highest viable number) pathogen present (fig 1). P aeruginosa was predominant in 15 samples, H influenzae in 11 samples, M catarrhalis in 6 samples, S pneumoniae in 4 samples, and Escherichia coli in the remaining 2 samples. The predominant pathogen could be isolated in all samples 24 h after collection, irrespective of storage temperature. When samples were stored at 4°C, the numbers of each pathogen type were lower than for samples stored at 20°C, and this difference for all samples confirmed a statistically significant decrease (p<0.004) following 24 h at 4°C (mean 4.22×108 cfu/ml, SEM 1.43) compared with those at 20°C (mean 1.15×109 cfu/ml, SEM 0.50). Viable numbers recovered following storage at 20°C showed no difference to those immediately after collection (mean 1.21×109 cfu/ml, SEM 0.35).
Effect of posting on recovery and quantitation of bacteria
All bacterial species recovered from 42 fresh samples were also isolated in corresponding aliquots of sputum that were processed 24 h later, following postage to the laboratory (fig 2). Overall, there was no significant difference in mean viable numbers in fresh and posted aliquots: 1.89×109 cfu/ml (SEM 0.77) and 1.02×109 cfu/ml (SEM 0.29), respectively.
In the 13 samples where P aeruginosa was the predominant organism, the mean numbers were similar for fresh and posted aliquots: 1.87×109 cfu/ml (SEM 1.37) and 1.40×109 cfu/ml (SEM 0.78), respectively. Similarly, in the 13 samples in which H influenzae predominated, mean numbers were 3.34×109 cfu/ml (SEM 2.17) initially and 8.94×108 cfu/ml (SEM 3.88) following postage. In the remaining 16 samples (10 with M catarrhalis, 4 with S pneumoniae and 1 each with E coli and H parainfluenzae), mean viable numbers were also the same immediately after collection and following postage to the laboratory: 8.35×108 cfu/ml (SEM 2.51) and 8.06×108 cfu/ml (SEM 2.52), respectively.
DISCUSSION
The need to process samples as soon as possible to circumvent erroneous results has been recommended because organisms such as H influenzae have fastidious growth requirements, whereas Pseudomonas spp. have a tendency to overgrow other organisms, and it has previously been recommended that samples are stored at 4°C.7
The current study showed that the predominant organisms could be isolated 24 h after collection following storage at 4°C and 20°C, although there was some variation in viable numbers. In sputum stored at 4°C there was a statistically significant reduction in viable numbers compared with immediately after collection. However, viable numbers following storage at 20°C were similar to those in the original sample.
A previous investigation into the effects of overnight refrigeration has also shown that this has an inhibitory effect on the growth of some bacteria.11 Thirty-eight of 131 (29%) isolates, including H influenzae, S pneumoniae, M catarrhalis and Gram-negative bacilli, were inhibited, and nine (6.9%) isolates were not recovered at all after refrigeration.11 Similar results have been shown by Gough who reported that in 8.7% of samples pathogens, including Haemophilus sp., S pneumoniae and M catarrhalis, failed to be recovered following storage at 4°C.9 In contrast to these findings, Wong and colleagues7 reported no detectable change in viable numbers of S aureus, P aeruginosa, H influenzae or S pneumoniae after storage at 4°C for up to 2 days. A subsequent investigation, however, into the effect of overnight refrigeration on bacterial growth from protected specimen brush cultures reported that of the four organisms studied only H influenzae showed a significant decrease in growth12; this supports the findings of the study reported here where viable numbers of four out of 11 (36.4%) H influenzae isolates were reduced at 4°C compared with only one out of 11 (9.1%) at 20°C
There have been very few studies of bacterial recovery following storage at 20°C, but it has been shown that S pneumoniae isolated in sputum from patients with pneumonia can be recovered for as long as 6 days from samples stored at room temperature.13 In the present study, sputum was stored for a maximum of 24 h only, as this is potentially the length of delay encountered in clinical practice. The data suggest that such a strategy has no effect on the subsequent results.
Therefore, contrary to previous recommendations, the results presented here suggest that if there is a delay, sputum should be stored at 20°C to prevent the decrease in numbers, especially H influenzae, observed following storage at 4°C. The observation that numbers remain stable at 20°C is important since it allows collection of samples from Primary Care Trusts and peripheral hospitals, and also reduces the associated costs of refrigerated storage and transport to a central laboratory base. This is supported by the results comparing fresh samples with those sent in the post. Posting was not found to affect the subsequent recovery of the bacterial species present. The bacteria isolated from fresh aliquots of all 42 samples were identical to those from posted aliquots, with no difference in the mean numbers. Numbers did vary by up to one order of magnitude in approximately 50% of the samples; however, this represented an increase and a decrease, and is likely to reflect differences between aliquots used, as reported previously,10 since the average value was unchanged.
The results of the study reported here do not support the previous observation that H influenzae and S pneumoniae are isolated less frequently from samples received via the postal system.5 This may be partly due to the culture techniques employed, or the fact that samples were stored in metal containers which may affect the viability of some species.
Transporting samples by post with little or no effect on qualitative and quantitative results has important implications. It has previously been used in our laboratory for studying sputum from adult patients with cystic fibrosis at a centre outside Birmingham, and results showed that following postage of an aliquot of each of 55 samples the isolation of Pseudomonas spp. and S aureus was similar in both centres, although greater numbers of H influenzae and M catarrhalis were recovered in the posted samples.14 This may be partly due to the different culture techniques used, but it does confirm that posting does not adversely affect isolation rate, even in samples where Pseudomonas spp. are present when they could potentially overgrow more fastidious bacteria.
Management of patients with bacterial colonisation requires knowledge of both the predominant species and the viable number present, since the latter drives the local inflammatory response.15 16 This is especially important during exacerbations when culture results are needed to guide decisions concerning current and future antibiotic treatment. Patients are often too unwell to attend specialist clinics and provide sputum during an exacerbation, and the ability to send samples to the laboratory may prove critical in patient management. The ability to provide culture results at the onset of an exacerbation at a distance will lead to more effective management of patients with chronic lung disease. The current study confirms that storage at room temperature and posting samples does not adversely affect results and hence decision making in the management of such patients.
In conclusion, although sputum should ideally be cultured as soon as possible, samples should be stored at room temperature if a delay is inevitable. In addition, bacterial culture performed on samples collected away from the laboratory and posted by first-class post to arrive the following day appears to be accurate both in terms of qualitative and quantitative results.
Take-home messages
If there is a delay in processing, sputum samples should be stored at room temperature and not refrigerated to prevent a decrease in numbers of fastidious bacteria such as Haemophilus influenzae.
Bacterial culture performed on sputum samples collected away from the laboratory and posted by first-class post to arrive the following day appears to be accurate both in terms of qualitative and quantitative results.
Acknowledgments
The authors would like to thank Dr Clare Newall for help with statistical analysis.
REFERENCES
Footnotes
Competing interests: None.