Article Text
Abstract
Objective Growing evidence suggests that endothelial injury is involved in the pathophysiology of chronic obstructive pulmonary disease (COPD). Circulating endothelial microparticles (EMPs) increase in patients with COPD because of the presence of endothelial injury. We examined the relationship between EMP number and changes in forced expiratory volume in 1 s (FEV1) in patients with COPD.
Design Prospective study.
Setting One hospital in Japan.
Participants A total 48 outpatients with stable COPD coming to the hospital from September 2010 to September 2011.
Primary and secondary outcomes measured Blood samples were collected and vascular endothelial (VE)-cadherin EMPs (CD144+ EMPs), E-selectin EMPs (CD62E+ EMPs) and platelet endothelial cell adhesion molecule EMPs (CD31+/CD41− EMPs) were measured using fluorescence-activated cell sorting. Annual FEV1 changes were evaluated using FEV1 data acquired a year before and a year after sample collection.
Results The number of E-selectin and VE-cadherin EMPs showed significant negative correlations with annual FEV1 changes (rs=−0.65, p<0.001, rs=−0.43, p=0.003, respectively). Leucocyte counts tended to be correlated with annual FEV1 changes, but this correlation was not significant (rs=−0.28, p=0.057). There were significant differences in annual FEV1 changes between with and without history of frequent exacerbation (p=0.006), and among Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages (p=0.009). Multiple linear regression analysis revealed E-selectin EMP to be the only significant parameter associated with annual FEV1 changes, independent of VE-cadherin EMP, GOLD stages, leucocyte counts, and history of frequent exacerbation. Receiver operating characteristic curves showed the optimum E-selectin EMP cut-off level for prediction of rapid FEV1 decline (>66 mL/year) to be 153.0/µL (areas under curve 0.78 (95% CI 0.60 to 0.89); sensitivity, 67%; specificity, 81%).
Conclusions The high E-selectin EMP levels in stable patients with COPD are predictive of rapid FEV1 decline.
Trial registration number UMIN000005168.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
Statistics from Altmetric.com
Strengths and limitations of this study
-
This is a prospective observational study investigating the relationship between endothelial microparticle (EMP) numbers and annual forced expiratory volume in 1 s (FEV1) changes in chronic obstructive pulmonary disease (COPD) patients.
-
High E-selectin EMP levels in stable condition predict rapid FEV1 decline independent of vascular endothelial (VE)-cadherin EMPs, GOLD stages, leucocyte counts, and history of frequent exacerbation.
-
A relatively small number of patients from a single institution.
-
We could not exclude influence of reduced pulmonary capillary bed in advanced COPD lungs on circulating EMP numbers.
Introduction
Chronic obstructive pulmonary disease (COPD) is a lung condition defined by airflow limitation. The severity of COPD is determined by the degree of airflow limitation, and disease progression is evaluated by a decline in forced expiratory volume in 1 s (FEV1). However, FEV1 decline is not a good parameter to judge COPD progression in the short term. Therefore, new biomarkers to evaluate disease activity and heterogeneity are needed.1 Growing evidence suggests that endothelial inflammation is closely involved in the pathophysiology of COPD. Endothelial impairment in the systemic vasculature, evaluated by flow-mediated dilation of the brachial artery, was reported to be associated with lower FEV1 values.2 Furthermore, the incidence of premature death due to cardiovascular diseases was found to be high in patients with decreased FEV1.3 Severe endothelial inflammation exists in patients with COPD with a history of frequent exacerbation, even during the stable phase,4 and frequent exacerbation is a well-known factor associated with rapid FEV1 decline.5–8 Therefore, we speculated that the evaluation of endothelial impairment may enable the prediction of COPD progression.
Circulating endothelial microparticles (EMPs) are shed membrane vesicles released from activated or apoptotic endothelial cells when these cells are injured.9 ,10 The number of EMPs was found to be significantly increased in patients with vascular disorders such as coronary heart disease,11 stroke,12 renal failure13 and hyperlipidaemia14 as well as in current smokers.15 These EMPs are recognised markers of endothelial damage and are defined according to endothelial-specific antigens on their membranes, such as CD144 EMPs (vascular endothelial (VE)-cadherin EMPs), CD31/CD41 EMPs (platelet endothelial cell adhesion molecule (PECAM) EMPs) and CD62E EMPs (E-selectin EMPs). Differences among these released EMP subtypes have been observed in patients with various diseases such as pulmonary hypertension and acute coronary syndrome16 ,17 during COPD exacerbation,4 and they reflect differences in the condition of injured endothelial cells or types of inflammation.18 ,19
We previously reported that, compared with healthy controls, the numbers of EMPs, mainly released from pulmonary capillary vasculatures, was significantly increased in patients with stable COPD, while it was further increased in patients with exacerbated COPD.4 In addition, Thomashow et al20 (the MESA group) also reported significant correlations between EMP number and the degree of lung destruction and emphysematous changes in patients with mild-to-moderate COPD and those without. Furthermore, apoptosis of pulmonary capillary endothelial cells was reported to lead to emphysematous changes in animal model.21 However, a relationship between elevated EMP numbers and COPD progression has not been clarified. Therefore, we hypothesised that high EMP levels predicted rapid FEV1 decline and conducted this prospective study to determine the correlation between EMP number and annual FEV1 changes in patients with COPD.
Some of the results from this study have been previously presented in an Abstract.22
Methods
Patient population
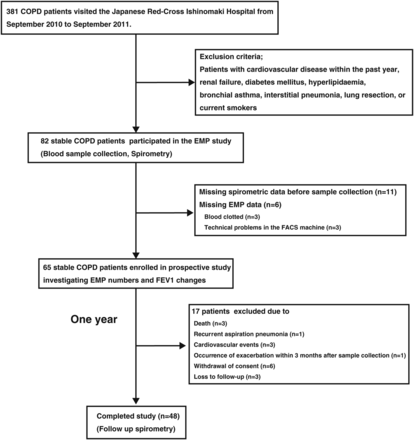
This was a prospective observational study investigating the relationship between EMP number and annual FEV1 changes in patients with COPD. The study protocol is summarised in figure 1. All participants provided written informed consent.
Flowchart of patient enrolment and reasons for exclusion. COPD, chronic obstructive pulmonary disease; EMP, circulating endothelial microparticle; FACS; fluorescence-activated cell sorting; FEV1, forced expiratory volume in 1 s.
Airflow limitation was determined by spirometry and defined as a postbronchodilator FEV1/forced vital capacity (FVC) of <0.70. Severity was classified in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.23 We defined stable patients with COPD as those without episode of exacerbation within 3 months prior to sample collection. We excluded participants with history of cardiovascular events such as acute coronary syndrome and acute stroke within the past year prior to sample collection, to exclude influences of these cardiovascular events on the EMP levels in stable patients with COPD.11 ,12 In addition, we excluded those with renal failure,13 diabetes mellitus,24 hyperlipidaemia14 and thromboembolic disease25 because these diseases reportedly influence EMP number. Current smokers were also excluded because the EMP number is reportedly increased in current smokers compared with that in never smokers.15 We excluded patients who had undergone lung resection because lung resection itself may influence annual FEV1 changes. Patients with COPD with other respiratory diseases such as asthma and interstitial pneumonia were also excluded. No patients had medication of statin. Among 381 patients with COPD who visited at the Japanese Ishinomaki Red Cross Hospital from September 2010 to September 2011, a total of 82 outpatients with stable COPD, including 53 patients enrolled in the farmer study published in Thorax 2012,4 at the Japanese Ishinomaki Red Cross Hospital were eligible to participate in the EMP study. Of these, 17 were excluded because of missing spirometric data before blood sample collection or EMP data, leaving 65 stable patients with COPD enrolled in this prospective study investigating EMP number and annual FEV1 changes. Blood samples and spirometry data were collected from all participants. After a year of observation, spirometry was repeated. An additional 17 patients were excluded during the observation period, leaving 48 that completed the study. No patient changed drug treatment 2 years before sample collection or during the observation period.
Blood sampling
Peripheral venous blood was collected into heparinised tubes. Blood samples were centrifuged for 10 min at 170 g, and plasma was then harvested and centrifuged for 20 min at 1500 g to obtain platelet-free plasma (PFP) as previously described.26
Characterisation of EMPs
EMP subpopulations were determined by flow cytometry in PFP according to the expression of membrane-specific antigens. Three EMP subtypes were defined as follows: CD144 (FITC) MPs (VE-cadherin EMPs), CD31 (FITC)/CD41 (PE) MPs (PECAM EMPs) and CD62E (PE) MPs (E-selectin EMPs). PFP (10 µL) was incubated with each specific antibody (see online supplementary table E1) for 30 min at room temperature. Samples were then diluted in 300 µL of a 0.9% saline salt solution. Equal volumes of sample and Flowcount beads (Beckman–Coulter) were then added and analysed using fluorescence-activated cell sorting (FACS). We made a microparticle gate to include events <1 µm in an FSC-SSC scattergram using 1 µm beads (Fluka, Sigma). Appropriate isotype control antibodies were used to increase the specificity of MP detection. The EMP numbers were calculated as absolute numbers of EMPs per µL PFP.
Pulmonary function tests
Spirometry measurements were conducted by a well-trained technician following the American Thoracic Society and European Respiratory Society guidelines after participants inhaled a bronchodilator prior to sample collection.27 Acceptable manoeuvres for spirometry measurements were defined as those with a sufficient peak expiratory flow, a rapid start, an absence of major flow fluctuations and an adequate duration of expiration. Pulmonary function testing was performed in duplicate, and the best FEV1 and FVC values were recorded from acceptable manoeuvres.28
Calculation of annual FEV1 changes
Annual FEV1 changes at sample collection were evaluated using FEV1 data obtained a year before sample collection, at sample collection and a year after sample collection. FEV1 data for each patient over 2 years were plotted on FEV1 versus year scattergrams. To evaluate annual FEV1 change, regression coefficients (mL/year) were calculated using JMP V.9 statistical analysis software (SAS Institute, Cary, North Carolina, USA).
Severity of emphysema evaluated by the Goddard classification
We visually evaluated the severity of emphysema according to the modified Goddard classification as previously described.29 We used a high-resolution chest CT to quantify low attenuation areas (LAAs). LAA was scored for the right and left sides of the upper, middle and lower lung fields. Zero represented no abnormality, while 1 was given for up to 25%, 2 for up to 50%, 3 for up to 75% and 4 for almost the total absence of normal lung tissue. The total possible scores ranged from 0 to 24. The evaluation was independently performed by two pulmonologist in a blinded fashion, and the means of the two scores assigned by the two readers was calculated.
Statistical analysis
All data are presented as mean±SD unless otherwise stated. Differences in annual FEV1 changes between sexes, history of frequent exacerbation, use of inhaled or systemic corticosteroids and use of β agonists were analysed using the Mann-Whitney U test. The Kruskal-Wallis test was used to compare annual FEV1 changes among the four GOLD stages. Correlations between annual FEV1 changes and EMP number, age, body mass index, pack-years smoking index, leucocyte counts and C reactive protein were calculated using Spearman non-parametric methods. Multivariate linear regression analysis was performed to evaluate the impact of VE-cadherin EMP number, E-selectin EMP number, GOLD stage and history of frequent exacerbation on annual FEV1 changes. We evaluated sensitivity, specificity and respective areas under the curve (AUC) using receiver operating characteristic (ROC) curves. The optimum cut-off value of the E-selectin EMP number for prediction of rapid FEV1 decline was calculated to maximise the sum of sensitivity and specificity by minimising the distance of the cut-off value to the top-left corner of the ROC curve. p Values of <0.05 were considered statistically significant. All analyses were performed using the JMP V.9.
Results
Characteristics of subjects who completed the study
The characteristics of the 48 stable patients with COPD at sample collection are shown in table 1. Eight patients were classified as stage I, 16 as stage II, 13 as stage III and 11 as stage IV. We defined frequent exacerbation as two or more episodes of exacerbation every year according to the GOLD guideline.23 LAA score ranged from 2 to 24 (median 16.5). There was significant correlation between LAA scores and FEV1/FVC ratio (rs=−0.36, p=0.015), FEV1 (rs=−0.31, p=0.035) or predicted FEV1% (rs=−0.41, p=0.005). A total of 22 patients had a history of frequent exacerbation. In the past year, prior to the sample collection, 46 episodes of exacerbation occurred in all 21 patients with history of frequent exacerbation, and there was no episode in patients without history of exacerbation. During 1-year follow-up period, 57 episodes occurred in 23 patients including of 21 patients with history of frequent exacerbation. All patients had used anticholinergics, 35 had used inhaled or systemic corticosteroids and 25 had used β agonists.
Characteristics of the 48 stable patients with COPD at the time of enrolment in the study
Rate of annual FEV1 changes
The mean rate of annual FEV1 changes was −32.5 ± 51.5 mL/year, which was consistent with that reported in a previous study performed by Vestbo et al6 (−33 ± 52 mL/year). We defined patients with rapid FEV1 decline as those in less than the 25th centile of annual FEV1 changes. There were 12 patients with rapid FEV1 decline, and annual FEV1 changes ranged from −66.0 to −151.0 mL/year (median, −84.5 mL/year).
Effects of sex, GOLD stage, history of frequent exacerbation or use of drugs on annual FEV1 changes (univariable analysis)
There was no significant difference in annual FEV1 changes between men and women (p=0.473). In addition, there was no difference between patients who used steroids or β agonists and those who did not (steroids: p=0.862, β agonists: p=0.861). In contrast, there was a significant difference among GOLD stages (p=0.009). FEV1 declined more rapidly in patients with frequent exacerbation than in those without (p=0.006; table 2 and figure 2).
Effects of categorical variables on the annual FEV1 changes in stable patients with COPD (univariable analysis)
Comparisons of annual forced expiratory volume in 1 s (FEV1) changes (A) among Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages and (B) between patients with and without a history of frequent exacerbation.
Correlation between annual FEV1 changes and EMP number or continuous variables of patient characteristics (univariable analysis)
There were significant negative correlations between annual FEV1 changes and VE-cadherin EMP (rs=−0.43, p=0.003) or E-selectin EMP number (rs=−0.65, p<0.001). However, there was no significant correlation among annual FEV1 changes and age, pack-years smoking index, leucocyte count or C reactive protein (table 3 and figure 3).
Effects of various continuous variables on annual FEV1 changes in stable patients with COPD (univariable analysis)
Correlations between annual FEV1 changes and (A) VE-cadherin EMPs, (B) E-selectin EMPs and (C) PECAM EMPs. Filled circles indicate patients with a history of frequent exacerbation and open circles indicate patients without a history of frequent exacerbation. EMPs, circulating endothelial microparticles; FEV1, forced expiratory volume in 1 s; PECAM, platelet endothelial cell adhesion molecule; PFP, platelet-free plasma; VE-cadherin, vascular endothelial-cadherin.
Independent parameters associated with annual FEV1 changes (multiple linear regression analysis)
Following the results of univariate analysis, multivariate linear regression analysis was performed with annual FEV1 decline as the dependent variable and VE-cadherin EMP number, E-selectin EMP number, GOLD stages and history of frequent exacerbation, and leucocyte counts as independent variables. Only E-selectin EMP number was identified as an independent parameter (|r|=0.67, p<0.001; table 4). In addition, E-selectin EMP number was an independent predictor for COPD exacerbation during the follow-up period (see online supplementary table E3).
Comparison of impact on annual FEV1 changes among VE-cadherin EMP number, E-selectin EMP number, GOLD stage and history of frequent exacerbation (multiple linear regression analysis)
ROC curve of E-selectin EMP number for the prediction of rapid FEV1 decline
We assessed the association of E-selectin EMP number under a stable condition with rapid FEV1 decline (≥66 mL/year) using AUC analysis. ROC curves showed that the optimum cut-off value of E-selectin EMP level for the prediction of rapid FEV1 decline was 153.0/µL (AUC 0.78 (95% CI 0.60 to 0.89; sensitivity, 67%; specificity, 81%; figure 4).
Receiver operating characteristic (ROC) curve of E-selectin endothelial microparticle number for prediction of rapid forced expiratory volume in 1 s decline.
Discussion
In this study, we found that VE-cadherin and E-selectin EMP number under a stable condition had significant negative correlations with annual FEV1 changes. In multiple linear regression analysis, only E-selectin EMP level under a stable condition was a significant factor associated with annual FEV1 changes, while VE-cadherin EMP level, GOLD stage and a history of frequent exacerbation were not. The ROC curve showed that the optimum cut-off value of E-selectin EMP level for the prediction of rapid FEV1 decline was 153.0/µL, with good sensitivity and specificity. From these results, we concluded that a high E-selectin EMP level under a stable condition predicted rapid FEV1 decline after a year in patients with COPD. Therefore, the E-selectin EMP number under a stable condition could be a good biomarker to predict the prognosis of patients with COPD.
Elevated E-selectin EMP numbers may suggest the presence of endothelial inflammation, even under a stable condition. We previously reported that E-selectin EMP numbers increased during COPD exacerbation but decreased to below baseline levels after the patient's condition recovered,4 suggesting that elevated E-selectin EMP numbers can be modulated by medical treatments. Although a treatment that decreases E-selectin EMP numbers has not yet been established, maintaining that a low E-selectin EMP level may be a possible strategy to prevent the progression of lung destruction and/or airway obstruction in COPD.
Previous studies indicated that frequent exacerbation is reportedly associated with rapid FEV1 decline5–8; however, a history of frequent exacerbation was not an independent factor for FEV1 decline in this study. One reason to explain this discrepancy is that effects of frequent exacerbation on FEV1 declines were weak when compared with other factors, such as smoking, and reportedly varied among studies due to differences in study design, sample size and inclusion criteria. In this study, E-selectin EMP level had a significant negative correlation with annual FEV1 changes in those without (rs=−0.60, p=0.002) and with a history of frequent exacerbation (rs=−0.50, p=0.027). In addition, E-selectin EMPs have significant positive correlations with leucocyte counts and SAA (see online supplementary table E2). Patients with COPD with a history of frequent exacerbation demonstrate high E-selectin EMP numbers under stable conditions,4 suggesting the presence of higher endothelial inflammation. Recent reports indicated that EMP itself bound with circulating leucocytes, which induced further inflammation.30 Therefore, we speculated that endothelial inflammation was a predominant factor for the rapid FEV1 decline in patients with COPD. E-selectin EMPs can be released from both the systemic and the pulmonary vasculature. The high levels of E-selectin EMPs could be from the systemic inflammatory response present in COPD. However, increased EMP levels correlated with early lung destruction in smokers,15 suggesting EMP release from injured pulmonary capillary endothelium. Further study to identify the origin of E-selectin EMPs is necessary.
PECAM EMP number was reportedly associated with the percent emphysema on CT scans,20 but it did not correlate with annual FEV1 decline in this study. PECAM-1 is constitutively expressed on endothelial cells, but E-selectin is rapidly expressed when endothelial cells are activated by inflammation.31 Therefore, the released E-selectin EMPs reflected the presence of endothelial inflammation. However, almost 90% PECAM EMPs in patients with COPD coexpressed annexin V,4 and released PECAM EMPs reflect apoptosis of endothelial cells.18 ,19 Therefore, elevated PECAM EMPs are the consequence of lung destruction with endothelial apoptosis, and endothelial inflammation with upregulated E-selectin expression dominantly plays a key role in prospective COPD progression.
A limitation of this study is that EMP number may change under certain conditions, particularly before the onset of clinical events and after treatments for the events. To clarify the relationship between changes in pulmonary function and EMP number, we excluded patients who suffered from exacerbations within 3 months after sample collection in this study. In addition, because the onset of cardiovascular diseases may influence EMP number, we excluded patients who suffered from these events within a year before and after sample collection. Even after these careful exclusions, however, obscure inflammation may exist and influence the number of circulating EMPs. Furthermore, we could not separate chronic inflammation as a feature of COPD and transit inflammation caused by minor infection or inhalation of air pollutants.
Another limitation is that we did not measure diffusing capacity of the lung for carbon monoxide (DLCO) in this study. DLCO is a useful early marker of capillary injury in smokers.15 Although current smokers were excluded from the study, it would be interesting to show DLCO values to strengthen the rationale of vascular studies in COPD and give hints to pathogenic mechanisms of endothelial injury in this study. Instead, we accessed the degree of lung destruction using LAA score. There was no correlation between EMP numbers and LAA scores in this study (data not shown), suggesting the small airway disease of COPD might be more relevant to increased EMPs. However, it is well known that the pulmonary capillary bed is significantly reduced in emphysematous regions, and this reduced number of endothelium depresses the circulating EMP numbers. Therefore, we could not discard the possibility of the relationship between increased EMPs and lung destruction.
Circulating EMPs can be repeatedly measured from patients’ blood, and the cost of the measurement is quite low. Therefore, EMPs are practical parameters for monitoring the condition of patients with COPD. However, some technical difficulties remain in the measurement of MPs.32 For example, the sensitivity of detecting MPs is different among various kinds of FACS machines, and differences in the protocol of the centrifugation also influence results of MP numbers. In addition, changes in E-selectin EMP numbers in patients with COPD are more rapid than those in other EMPs.4 Therefore, time points of withdrawing patients’ blood should be fixed. Standardisation of the protocol is essential to perform reliable analyses in a large multicentre study.
In conclusion, we found that high E-selectin EMP numbers under stable conditions predict rapid FEV1 decline in patients with COPD. Standardising reliable protocol of the EMP enumeration is technically challenging because the size of EMPs is near the detection limit of flow cytometers. However, the E-selectin EMP number sensitively and rapidly reflects changes in disease progression and may be a good biomarker of COPD.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
-
Contributors TT and HK participated in conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript. SKobayashi and MYanai participated in provision of study material and patients, obtain informed content from the patients and final approval of manuscript. NF, TS, CO, YT, MYamada participated in collection and assembly of data, data analysis and interpretation, and final approval of manuscript. MYamaya, SKurosawa and MYamauchi participated in collection and assembly of data, final approval of manuscript.
-
Funding This work was supported by a grant from the Japan Society for the Promotion of Science (No. 22390163 and 25293190) to HK.
-
Competing interests None.
-
Ethics approval The study was approved by the Ethics Committee of the Japanese Red Cross Ishinomaki Hospital, Japan, and registered at the University Hospital Medical Information Network Clinical Trials Registry (Clinical Trial Number: UMIN000005168).
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Data sharing statement No additional data are available.