-
PDF
- Split View
-
Views
-
Cite
Cite
Kiyoko Kawamura, Kenzo Hiroshima, Takeo Suzuki, Kuan Chai, Naoto Yamaguchi, Masato Shingyoji, Toshikazu Yusa, Yuji Tada, Yuichi Takiguchi, Koichiro Tatsumi, Hideaki Shimada, Masatoshi Tagawa, CD90 Is a Diagnostic Marker to Differentiate Between Malignant Pleural Mesothelioma and Lung Carcinoma With Immunohistochemistry, American Journal of Clinical Pathology, Volume 140, Issue 4, October 2013, Pages 544–549, https://doi.org/10.1309/AJCPM2Z4NGIIPBGE
Close - Share Icon Share
Abstract
To pathologically distinguish mesothelioma from lung carcinoma, particularly adenocarcinoma.
We conducted immunohistochemical analyses on clinical specimens, including 26 cases of mesothelioma, 28 cases of lung adenocarcinoma, and 33 cases of lung squamous cell carcinoma.
We found that CD90 expression was useful in making a differential diagnosis between epithelioid mesothelioma and lung adenocarcinoma, whereas sarcomatoid mesothelioma and lung carcinoma specimens, irrespective of the histologic types, were negative in general. The sensitivity and specificity of CD90 expression in epithelioid mesothelioma and lung adenocarcinoma were comparable to those of well-established markers used for the differential diagnosis.
These data collectively indicate that CD90 is a novel diagnostic marker that contributes to a diagnosis of epithelioid mesothelioma.
Malignant mesothelioma is often associated with asbestos exposure and remains intractable despite recent treatment modalities.1 No procedure is currently available to prevent mesothelioma development after asbestos exposure, and the patient numbers will increase in industrialized and newly developing countries in the next decades.2 Mesothelioma is histologically classified into 3 categories—epithelioid, sarcomatoid, and biphasic types—and the epithelioid type is the major type among them. A differential diagnosis between mesothelioma, especially the epithelioid type, and lung carcinoma, particularly adenocarcinoma that invades into pleura, is often difficult in terms of surgical pathology but is quite important from the standpoint of therapeutic procedures. Previous studies demonstrated that immunohistochemical staining with a panel of various kinds of antibody (Ab) was valuable for diagnostics,3–7 which used calretinin, D2-40, and Wilms tumor product 1 (WT-1) molecules as a positive marker. Recently, Amatya et al8 showed that caveolin 1 could be a marker to differentiate epithelioid mesothelioma and lung adenocarcinoma, but contradictory data were also reported.9 It is thereby important to identify other candidate molecules to increase diagnostic accuracy. In this study, we found that CD90, expressed mainly in immunological and nervous systems, was positive for human mesothelioma cell lines but negative for lung carcinoma. We then demonstrated that immunohistochemical staining of CD90 was useful to differentiate between mesothelioma, particularly the epithelioid type, and lung carcinoma.
Materials and Methods
Cells
Human mesothelioma cells (NCI-H2452, NCI-H2052, NCI-H226, NCI-H28, and MSTO-211H) and mesothelium-derived Met-5A cells that were immortalized by the SV40 T antigen10 were obtained from American Type Culture Collection (Manassas, VA). Human lung squamous cell carcinoma cells (PC1, PC10, and QG56), human lung adenocarcinoma cells (PC9 and PC14), and human small cell lung carcinoma cells (QG90) were from Cell Resource Center for Biomedical Research, Tohoku University, Sendai, Japan.
Cell Surface Expression of CD90
Cells were stained with anti–CD90 Ab (Calbiochem, Darmstadt, Germany) followed by fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse immunoglobulin G (IgG) Ab (SouthernBiotech, Birmingham, AL) or with FITC-conjugated second Ab alone and were analyzed with FACSCalibur and CellQuest software (BD Biosciences, San Jose, CA).
Immunohistochemical Staining
Twenty-six mesothelioma samples were collected from clinical specimens between 2000 and 2008 at Chiba University Hospital, Chiba Rosai Hospital, or Chiba East Hospital, Japan. The diagnosis was based on a combination of clinical findings, imaging analyses, and gross observations at surgery and on pathologic examinations. The histopathologic diagnosis was confirmed by several immunohistochemical staining results with Ab against calretinin, WT-1, D2-40, cytokeratin 5/6, cytokeratin CAM5.2, cytokeratin AE1/AE3, carcino-embryonic antigen, thyroid transcription factor 1, desmin, smooth muscle actin, and S100.2,3,6,8 We also collected 28 lung adenocarcinoma and 33 lung squamous cell carcinoma specimens that were subjected to surgical resection at Chiba University Hospital between 2002 and 2004. Formalin-fixed, paraffin-embedded clinical specimens sliced at 4 μm thick were incubated with anti–CD90 Ab (AbD Serotec, Düsseldorf, Germany), followed by peroxidase-conjugated goat anti–mouse IgG Ab (Nichirei Biosciences, Tokyo, Japan) and developed with 3,3′-diaminobenzidine according to the manufacturer’s instructions (Nichirei Biosciences). We judged the specimens as positive when more than 10% of the tumor cells were stained with the anti–CD90 Ab.4
Results
Expression of CD90 on Mesothelioma and Lung Carcinoma Cells
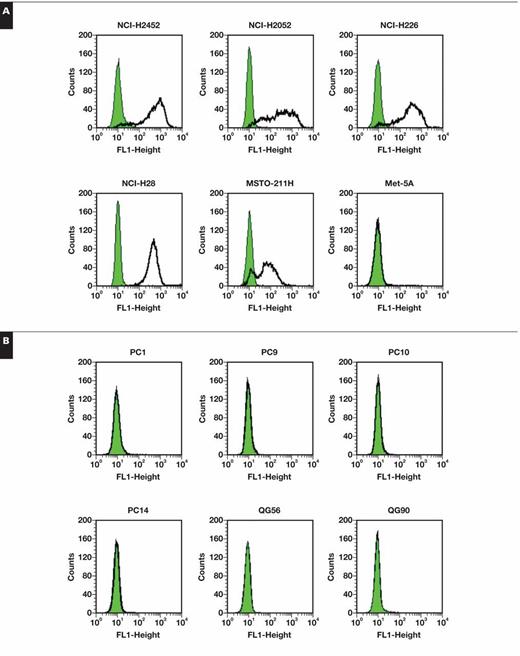
We examined 5 mesothelioma and 6 lung carcinoma cells for CD90 expression with flow cytometry Figure 1. All mesothelioma cells were positive for CD90, but all lung carcinoma cells tested were negative. MSTO-211H cells showed a biphasic staining pattern with a CD90-positive population as the majority. Interestingly, immortalized Met-5A cells of mesothelium origin were negative for CD90 expression.
CD90 Immunohistochemical Staining
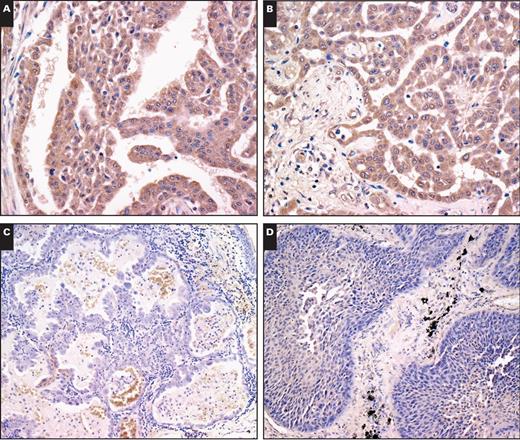
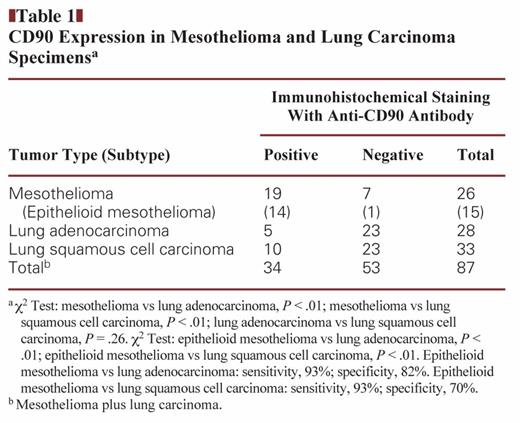
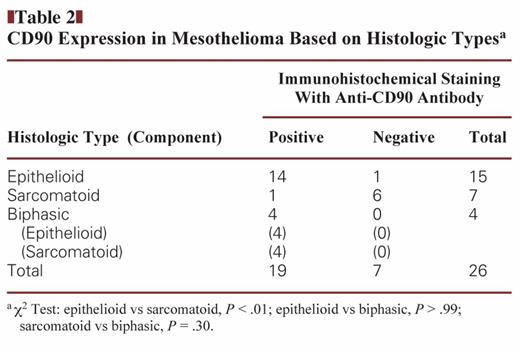
We examined clinical specimens of mesothelioma and lung carcinoma for CD90 expression Image 1. We tested 26 cases of mesothelioma, including 15 epithelioid, 7 sarcomatoid, and 4 biphasic specimens, and 61 cases of lung carcinoma, including 28 adenocarcinoma and 33 squamous cell carcinoma specimens Table 1. CD90 expression was detected in cytoplasmic portions with a granular manner. Most mesothelioma specimens were positive for CD90, whereas lung carcinoma samples, irrespective of histologic type, were CD90 negative (P < .01). There was no statistical difference in CD90 expression between adenocarcinoma and squamous cell lung carcinoma (P = .26). We also classified mesothelioma according to histology and found that almost all epithelioid mesothelioma specimens were positive for CD90, whereas sarcomatoid types were in general CD90 negative Table 2. All biphasic types were CD90 positive in both epithelioid and sarcomatoid components, but the sample numbers were limited. We also compared the CD90-positive rate between epithelioid mesothelioma and lung adenocarcinoma or lung squamous cell carcinoma and found that the positivity was significantly greater in epithelioid mesothelioma than in lung carcinoma irrespective of the histologic types (Table 1). Furthermore, the sensitivity and specificity for CD90 expression were 93% and 82%, respectively, between epithelioid mesothelioma and lung adenocarcinoma and were 93% and 70% between epithelioid mesothelioma and lung squamous cell carcinoma. These data suggest that CD90 is a valuable marker for immunohistochemistry to differentiate epithelioid mesothelioma from lung carcinoma.
Discussion
We demonstrated in this study that CD90 was expressed in mesothelioma but not in lung carcinoma cell lines and that CD90 is a valuable marker to differentiate epithelioid mesothelioma from lung carcinoma. The differential pathologic diagnosis between epithelioid mesothelioma and lung carcinoma is often difficult, particularly in the case of adenocarcinoma that has developed adjacent to the pleura. Expression of CD90 in mesothelioma has not been well described, although a number of immunohistochemical analyses have been performed with clinical specimens.4–8 A genome-wide gene expression analysis did not identify CD90 as a potential marker for mesothelioma,11 but characterization of established mesothelioma cell lines showed that CD90 was one of the molecules expressed in mesothelioma.12 CD90 is also known as one of the candidates for cancer stem cell markers,13 and the present study showed that immortalized Met-5A cells of mesothelium origin were negative for CD90 expression. Our study, however, also showed that human immortalized fibroblasts, HFF14 and OUMS-24,15 were positive for CD90, and 3 of 5 reactive mesothelial hyperplasia specimens expressed CD90 with the same staining pattern as found in mesothelioma (data not shown). In contrast, 6 cases of normal mesothelium were negative for CD90 expression (data not shown). These data collectively imply that CD90 is not a representative marker for so-called stemness in mesothelioma, although a side population of mesothelioma seems to express CD90.16 Recently, Ziegler et al17 reported that CD90 could be a candidate protein marker to discriminate between pleural mesothelioma and lung adenocarcinoma based on their survey of proteomics for N-linked glycoprotein. They, however, examined CD90 expression just at the transcriptional level as their test for validity and showed only 2 respective clinical specimens with immunohistochemistry. In contrast, to our knowledge, our study is the first to reveal CD90 expression frequency with clinical specimens of mesothelioma and lung carcinoma.
Cell surface staining profiles of CD90 analyzed with flow cytometry on human mesothelioma and Met-5A cells (A) and human lung carcinoma cells (B). Bold lines and shaded areas show staining with anti-CD90 antibody (Ab) and second Ab alone, respectively.
Representative immunohistochemical staining of clinical specimens. Two different samples of epithelioid mesothelioma (A, ×20; B, ×20), lung adenocarcinoma (C, ×10), and lung squamous cell carcinoma (D, ×10) were stained with anti-CD90 antibody.
A diagnostic value of CD90 needs to be compared with other markers. Four major studies4,5,6,8 dealing with molecules that could differentiate epithelioid mesothelioma from lung adenocarcinoma examined the sensitivity and specificity of respective molecules Table 3. King et al5 investigated 88 published studies, Yaziji et al4 65 epithelioid mesothelioma and 22 lung adenocarcinoma cases, Kushitani et al6 90 epithelioid mesothelioma and 51 lung adenocarcinoma cases, and Amatya et al8 80 cases of both kinds of tumors, including 20 mesothelioma of nonpleural origin. The number of specimens in the present study was not as large as in previous studies, but we showed that immunohistochemical staining of CD90 attained a similar level for the diagnostic values in terms of sensitivity and specificity, as demonstrated with calretinin, D2-40, and WT-1. These data suggest that CD90 could be integrated as one of the Ab marker panels for mesothelioma diagnostics. We examined 14 samples among CD90-tested mesothelioma cases for the expression of calretinin, D2-40, and WT-1. The respective antigen-positive rates were not different from each other (calretinin, 71%; D2-40, 77%; WT-1, 71%; and CD90, 71%). We, however, found that 4 cases were CD90 positive among 7 cases that were negative for calretinin, WT-1, or D2-40; furthermore, 2 cases were CD90 positive among 3 cases that were positive for only 1 of the 3 conventional markers. In contrast, only 1 case was CD90 negative among 7 cases that were positive for all markers. The results suggest that distribution of CD90-expressed mesothelioma is different from that of mesothelioma expressing the conventional markers and that CD90, as a positive marker, would be helpful to detect the marker-negative mesothelioma.
Sensitivity and Specificity of Positive Immunohistochemical Markers That Distinguished Epithelioid Mesothelioma From Lung Adenocarcinoma in Previous Reports
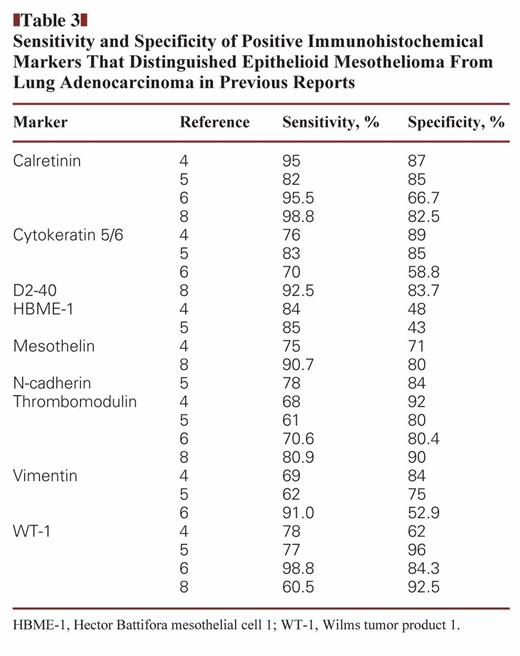
Sensitivity and Specificity of Positive Immunohistochemical Markers That Distinguished Epithelioid Mesothelioma From Lung Adenocarcinoma in Previous Reports

Immunohistochemical differentiation between epithelioid mesothelioma and lung squamous cell carcinoma has not been well investigated, but Ordóñez18 demonstrated that calretinin, mesothelin, and WT-1 are reliable positive markers based on his analysis with 30 respective cases. The sensitivity of calretinin, mesothelin, and WT-1 in his study was calculated as 100%, 100%, and 93%, respectively, and the specificity was 60%, 73%, and 100%, respectively. CD90 is thereby comparable to those molecules as a positive marker in immunohistochemistry that can differentiate epithelioid mesothelioma from lung squamous cell carcinoma. Interestingly, CD90 expression is almost negative in sarcomatoid mesothelioma, although sarcomatoid components of biphasic mesothelioma are CD90 positive. This could be partly due to putative etiologic differences between sarcomatoid mesothelioma and biphasic mesothelioma, but further investigations are required to confirm the CD90 reactivity with more clinical specimens.
The biological significance of CD90 expression in mesothelioma remains uncharacterized. CD90 molecules are primarily expressed in immunological and neurological systems with the glycosylphosphatidylinositol anchor, with knockout mice and other experimental systems showing that CD90 molecules have multiple roles in cell adhesion, apoptosis, and migration depending on experimental systems, as well as in neurite outgrowth and immunological activities.19 Moreover, decreased expression of CD90 levels in lung fibroblasts has increased fibrogenesis probably through transforming growth factor β signaling, demonstrating a possible crosstalk of CD90 with other signaling systems.20 CD90 is a soluble protein, and inflammatory responses can increase the secretion.20 Detecting CD90 molecules in pleural effusion thereby can be a diagnostic method for epithelioid mesothelioma, and CD90 can also be a target of mesothelioma treatments, such as in the case of immunotherapy. In conclusion, CD90 is a novel marker for mesothelioma diagnosis and has a comparable ability to other conventional markers to differentiate epithelioid mesothelioma from lung carcinoma, particularly adenocarcinoma.
This study was supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the grant-in-aid for Research on Seeds for Publicly Essential Drugs and Medical Devices from the Ministry of Health, Labor and Welfare of Japan; and a grant-in-aid from the Nichias Corporation.
References