-
PDF
- Split View
-
Views
-
Cite
Cite
Shigeki Fujitani, Victor L. Yu, Quantitative Cultures for Diagnosing Ventilator-Associated Pneumonia: A Critique, Clinical Infectious Diseases, Volume 43, Issue Supplement_2, September 2006, Pages S106–S113, https://doi.org/10.1086/504488
Close - Share Icon Share
Abstract
The diagnosis of ventilator-associated pneumonia has been clouded by uncertainty, because a reference standard has never been established. The use of invasive procedures to obtain respiratory tract samples for culture, with quantitation of the bacteria isolated, has been the approach most commonly advocated. Quantitation of bacteria from lower respiratory tract specimens can be used to distinguish colonization from infection. We review the invasive procedures (bronchoalveolar lavage, protected specimen brushing, nonbronchoscopic bronchoalveolar lavage, and blinded bronchial sampling), the methods of quantitation used, the types of catheters used, the sample collection methods, and the criteria used as cutoffs for the quantitative cultures. Quantitation of lower respiratory tract samples is inherently unstable from a mathematical perspective, given the variability in the volume of fluid instilled and reaspirated and the magnitude and complexity of the area being sampled. We also briefly review the use of quantitation for bacterial infections other than pneumonia, including urinary tract infection and catheter-related bacteremia. The variability in both the methods and reference criteria in the studies reviewed show that the quantitation approach is neither standardized nor evidence based.
A reference standard for ventilator-associated pneumonia (VAP) has never been clearly established. Application of invasive procedures to obtain respiratory tract samples for culture, with quantitation of the microorganisms isolated, has been the most common approach advocated by investigators. The invasive procedures, including bronchoalveolar lavage (BAL) and protected specimen brushing (PSB), are used to obtain specimens from the lung that can be cultured. Quantitative criteria with defined cutoffs have been used to define the presence of infection. The benchmark study was performed by Fagon et al. [1], who enrolled 413 patients in a multicenter, randomized trial. A noninvasive management strategy (clinical criteria and isolation of organisms by nonquantitative analysis of endotracheal aspirates) was compared with an invasive management strategy of direct examination of bronchoscopic BAL or PSB specimens with quantitative cultures. Patients randomized to the invasive strategy group experienced significantly fewer deaths and decreased antibiotic use, compared with patients randomized to the noninvasive strategy group.
Other less invasive approaches for obtaining lower respiratory tract specimens, including blinded bronchial sampling and nonbronchoscopic BAL (also called mini-BAL, protected BAL, nonbronchoscopically collected BAL, nondirected bronchial lavage, and plugged telescoping catheter), were subsequently developed [2]; these procedures all continued the application of quantitative cultures in an attempt to distinguish colonization from infection. Mini-BAL is defined as nonbronchoscopic BAL with a lavage volume ⩽25 mL.
The favored approach today in resolving medical issues of uncertainty is to use evidence-based studies. However, an in-depth examination of the numerous studies that have used and evaluated these less-invasive approaches indicates that these studies do not fulfill the criteria of evidence-based medicine. This is not the fault of the innovative researchers who performed these studies, because technical advances for these newer procedures, such as mini-BAL, occurred before a respectable database could be accumulated for any single procedure.
The obvious reference standard of isolation by culture from biopsy or autopsy lung specimens is not easy to fulfill. Isolation of pneumonic pathogens at other nonsterile sites, such as blood or pleural fluid, is infrequent. Even these standards are not wholly satisfactory, because autopsy, histologic assessment, and culture of lung aspirates can also give misleading results [3–6]. This issue has been comprehensively reviewed by Fagon [1], Baselski and Wunderink [7], and Wunderink [8], who listed methods of fluid retention and specimen handling, thresholds for interpretation, methodology for smear preparation, and reproducibility that had not clearly been standardized. Campbell [9] reviewed 15 studies evaluating the accuracy of blinded invasive methods; variability was seen for the test description, quality of the sample, and receipt of antibiotics before the procedure. A meta-analysis of studies of PSB, BAL, and endotracheal aspiration found design-related bias for patient selection, BAL volume, and prior antibiotic therapy in evaluations of these procedures [5].
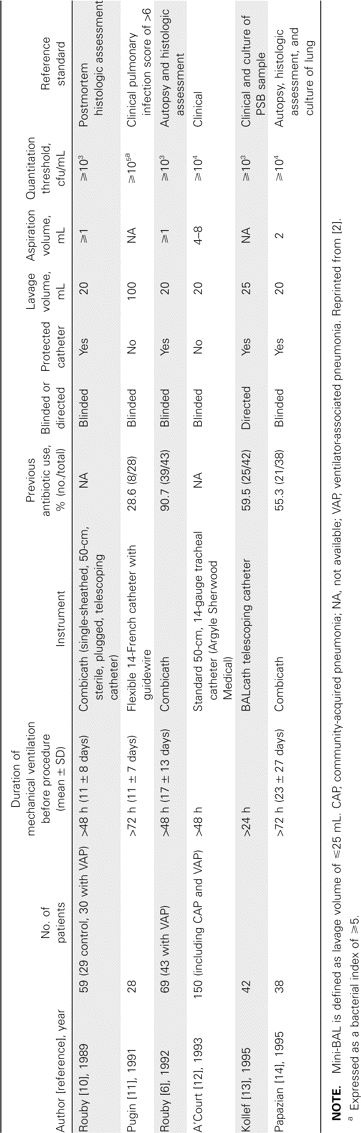
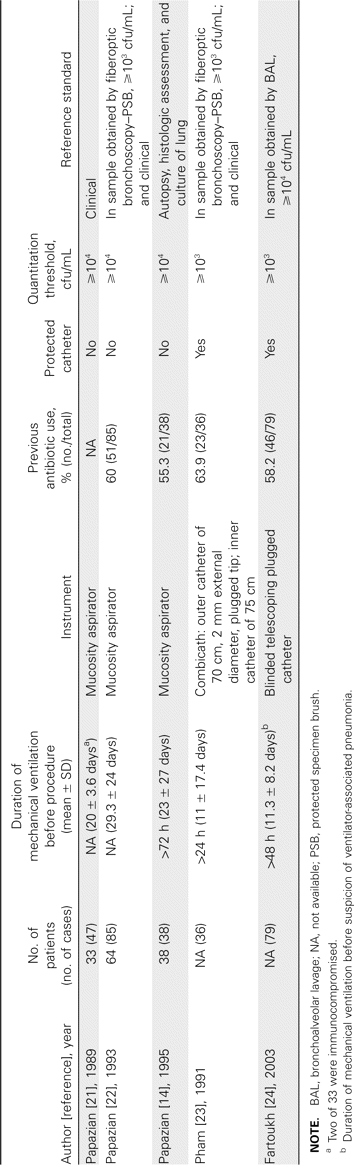
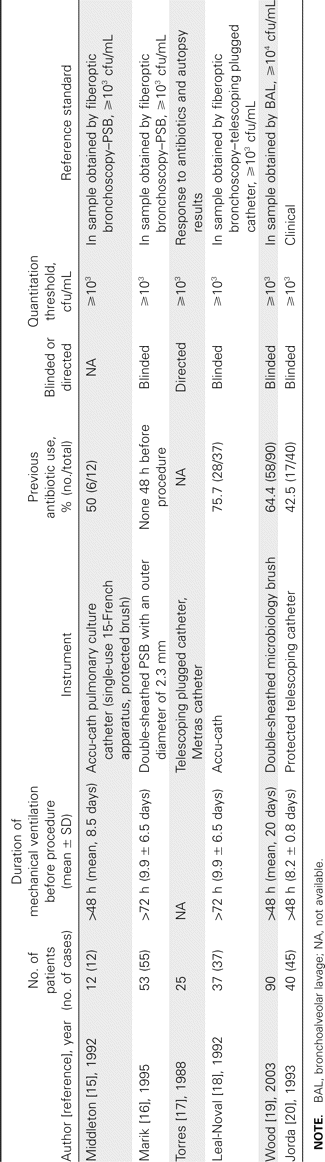
Quantitation of bacteria isolated from respiratory tract culture specimens has been applied for the less-invasive diagnostic procedures, including nonbronchoscopic BAL, blinded PSB, and blinded bronchial sampling [2]. In this review, we focus on the factor of quantitation. We reviewed prospective studies that reported explicit data for quantitation criteria used for diagnosis of VAP. We reviewed 17 studies (1 study overlapped): 7 for nonbronchoscopic BAL, including mini-BAL; 6 for PSB; and 5 for blinded bronchial sampling (tables 1–3).
Differences in methodology in comparative evaluation of nonbronchoscopic bronchoalveolar lavage (BAL), including mini-BAL.
Differences in methodology in comparative evaluation of blinded bronchial sampling.
Even within a given procedure, such as nonbronchoscopic BAL, blinded PSB, or blinded bronchial sampling, the catheters and instruments used were different (tables 1–3). Sample collection was variable, including blinded versus directed and protected versus nonprotected (table 1). For mini-BAL, the volumes of fluid for instillation and retrieval by aspiration were highly variable. Four studies instilled 20 mL, but the retrieval volume by aspiration ranged from ⩾1 mL to 4–8 mL (table 1). The remaining 2 studies used different instillation volumes, ranging from 25 to 100 mL; the retrieval volumes were unknown. The reference standard, as previously discussed, was variable; some studies used clinical diagnosis, some used results from BAL and PSB, and some used autopsy. In addition to the issue of circular reasoning, there was also variability in the quantitative culture results used for the reference standards for bronchoscopic BAL and bronchoscopic PSB. Not surprisingly, the sensitivity and specificity were highly variable [9]. However, a fatal flaw, in our opinion, was that, in 15 of a total of 16 studies, patients who were receiving antibiotics before and at the time of the procedure were not excluded. Several studies have noted that concurrent use of antibiotics lowers sensitivity [25–27].
Because of the invasiveness of the aforementioned procedures and their accompanying logistic problems, the use of endotracheal aspiration, a simpler procedure, to diagnose VAP remains common. Nine studies in which culture samples were obtained by endotracheal aspiration were reviewed by Cook and Mandell [28]. Unfortunately, in all 9 studies, patients who were receiving antibiotics when endotracheal aspiration was performed were not excluded. Three studies used qualitative cultures for diagnosis of VAP [29–31]; the remaining 6 studies used quantitative cultures [4, 25, 32–35]. The threshold varied from >105 cfu/mL [32, 34] to >106 cfu/mL [4, 25, 33, 35]. The reference standards for VAP were variable: 4 studies used clinical diagnosis [25, 32, 34, 35]; 1 study used PSB or culture of blood or pleural fluid, serologic analysis, or open lung biopsy [31]; 1 study used clinical diagnosis and PSB or BAL [33]; and the remaining 3 studies used autopsy with or without clinical diagnosis [4, 29, 30]. Cook and Mandell [28] concluded that the data compiled were so diverse that studies on the use of endotracheal aspiration were insufficient to generate clinical policy recommendations.
Here, we focus on the specific issue of quantitation; that is, the notion that quantitation of bacteria from lower respiratory tract specimens can be used to distinguish colonization from infection. This is the critical linchpin in the justification for using invasive procedures to obtain specimens through an airway that is normally colonized with bacteria. Given the necessity of an endotracheal tube for mechanical ventilation, colonization of the trachea by oropharyngeal bacterial flora is expected. Quantitation of the bacteria isolated has been proposed as a criterion to distinguish colonization from true infection [36, 37].
Quantitative results are usually reported in colony-forming units per milliliter. This mathematical relationship becomes unstable if the amount of fluid instilled and retrieved is variable. For example, if only a small volume of fluid is retrieved, then the bacterial count can be artifactually higher because of the lesser volume of diluent. On the other hand, if a greater volume of fluid is retrieved by aspiration, the concentrations will be lower because of the increased dilution. For mini-BAL, when the volume aspirated is only 1 mL, the range of variability of quantitation will be very large. This becomes a major problem because the quantitative inoculum is a bacteriologic continuum. In one study of PSB, ∼40% of respiratory specimens showed an increase from below the threshold for diagnosis of pneumonia to above the threshold a few days later [38].
In summary, the use of invasive procedures to diagnose VAP is currently based on quantitation—a method that is inherently unstable, given the crudeness of the approach and the magnitude and complexity of the area being sampled. The alveolar surface area distal to a wedged bronchoscope is 100 times greater than that of the peripheral airway, and ∼1 million alveoli (1% of the lung surface) are sampled, with ∼1 mL of actual lung secretions aspirated from the total lavage fluid [7]. We believe that the pitfalls described have made the entire approach futile.
If quantitation has not been validated for bronchial secretions in the diagnosis of VAP, has it been validated for any infection? Of note, quantitation has also not proven to be particularly accurate for diagnosis of other bacterial infections.
Urinary Tract Infection
Quantitation for diagnosis of urinary tract infection is used to distinguish infection from colonizing bacteria in the urethra or introitus passing into the voided urine. Traditionally, a quantitative culture of urine showing a bacterial concentration of ⩾105 cfu/mL has been considered to represent infection as opposed to colonization or contamination. Kass's [39] original protocol called for 3 consecutive clean-catch specimens for maximal sensitivity.
The utility of this technique in management has many caveats. Approximately 50% of noncatheterized patients with symptoms of urinary tract infection have colony counts in the bladder that are considerably lower than 105 cfu/mL [40, 41]. Slow-growing microorganisms may not reach counts of 105 cfu/mL. In catheterized patients, lower counts, of 102 cfu/mL, were predictive of infection [42, 43]. In patients with urinary tract infection, the counts will ultimately reach the higher threshold of 105 cfu/mL, if untreated; at that time, administration of antibiotics can still cure the infection, with little morbidity and no mortality. This wide margin of safety for undertreating cystitis is unlike the situation for VAP, in which outcome correlates directly with timely as well as appropriate antibiotic therapy.
Catheter-Related Bacteremia
A comprehensive review of bacteremia [44] indicated that, although a correlation has been observed for severity of disease and bacterial density, flaws in methodology and in the interpretation of quantitative cultures have discouraged widespread use. As a result, differential time to positivity, determined using automated blood culture systems, is now being evaluated as a more precise measurement for diagnosis of catheter-related bacteremias [45–48]. Unfortunately, the reference standard used to validate this approach included the traditional criterion of quantitation [47].
A major advantage of the use of quantitative cultures of blood and urine is that these sites are normally sterile, unlike the respiratory tract, in which oropharyngeal tracheal secretions are colonized with commensal bacteria, making the differentiation of a threshold that much more difficult.
In essence, there is no bacterial infection in which the use of quantitation has clearly proven to be satisfactory, as has been evidenced by validation studies (although quantitation has proven useful for defining infection by various viruses and parasites). Its use for cultures of respiratory tract secretions has more shortcomings than does its use for cultures from blood (endocarditis or catheter-related bacteremia vs. contamination) or urine (cystitis vs. contamination). Moreover, the studies of validation of its use in VAPs are inherently flawed, because the reference standards also used quantitative cultures as a criterion for infection [4]; this is obviously circular reasoning.
Summary
Tables 1–Tables 3 summarize the tremendous variability among studies evaluating procedures to distinguish colonization from infection. The methodologies are highly variable, the thresholds of quantitation are different, and the reference standards are a hodgepodge of clinical, microbiological, and histological criteria. We concluded in a previous commentary [2] that unless one is a researcher with a particular attachment to one invasive procedure, it is rational to use the least invasive procedure. Two limited studies of cultures of endotracheal aspirates versus cultures of samples obtained by invasive methods found no impact on mortality or clinical response [19, 49]. Light [50], stating that bronchoscopic BAL and PSB specimens are merely variable dilutions of the endotracheal aspirate, concluded that specimens obtained from locations only 5–15 cm apart along a patient's airway are unlikely to have substantially different bacteria. Thus, the use of culture of endotracheal aspirates without quantitation can easily be justified in the management of VAP.
A technological advance, either in imaging or a new molecular methodology, such as detection of microbial products (e.g., soluble triggering receptor expressed on myeloid cells) [51], may provide a far more accurate diagnostic tool for VAP than do today's crude attempts using quantitation with all of its pitfalls. The advent of transesophageal echocardiography revolutionized the diagnosis of endocarditis, making the use of quantitative cultures of blood obsolete. As newer methods for diagnosis of VAP continue to be developed, we predict that the use of quantitative cultures will someday be looked on as a curious but unsuccessful attempt at defining VAP.
Acknowledgments
Potential conflicts of interest. S.F. and V.L.Y.: no conflicts.
References
Figures and Tables
Differences in methodology in comparative evaluation of nonbronchoscopic protected specimen brushing (PSB).





Comments