-
PDF
- Split View
-
Views
-
Cite
Cite
Kris K. Oursler, Richard D. Moore, William R. Bishai, Susan M. Harrington, Diana S. Pope, Richard E. Chaisson, Survival of Patients with Pulmonary Tuberculosis: Clinical and Molecular Epidemiologic Factors, Clinical Infectious Diseases, Volume 34, Issue 6, 15 March 2002, Pages 752–759, https://doi.org/10.1086/338784
Close - Share Icon Share
Abstract
Using restriction fragment–length polymorphism data, we conducted a retrospective cohort study of 139 adult patients with pulmonary tuberculosis to investigate the clinical impact of Mycobacterium tuberculosis infection with a clustered isolate. The cumulative all-cause mortality rate during treatment was 21%. Patients with clustered DNA fingerprint patterns had a reduced risk of death, compared with patients with unique patterns (hazard ratio [HR], 0.5; 95% confidence interval [CI], 0.2–1.1), but this finding was confounded by age (adjusted HR, 0.8; 95% CI, 0.4–1.8). After adjustment for age, the strongest predictors of death were such underlying illnesses as diabetes mellitus, renal failure, chronic obstructive pulmonary disease, and human immunodeficiency virus infection. We conclude that comorbidity and immunosuppression are important predictors of survival for patients with pulmonary tuberculosis in an inner-city cohort. Recently transmitted infection, as determined by use of DNA fingerprinting to classify patients' isolates as being either clustered or unique, was not independently associated with death.
Molecular typing techniques have been used in outbreak investigations to identify Mycobacterium tuberculosis isolates in individuals who develop active tuberculosis (TB) within several years of infection [1–3]. Although estimates may be affected by incomplete sampling [4], the definition of clusters [5], and the extent of crowding [6], it is thought that 30%–50% of the isolates are clustered, according to matching DNA fingerprints [7–10]. Clustered isolates are thought to represent recent infection with rapid progression to disease in urban settings. Outbreak strains may achieve evolutionary success because of increased infectivity, enhanced virulence, reduced drug susceptibility, or host factors shared by the exposed individuals. It has been argued that an outbreak of TB that involved the strain CDC1551 was caused by either increased virulence [2] or increased transmissibility [11]. In vitro experiments have suggested that this strain is unique in its capacity to induce a vigorous host immune response [12]. In outbreaks that have been defined by restriction fragment–length polymorphism (RFLP) typing, factors that may blunt the host response, such as HIV infection [5, 7], injection drug use, and alcohol abuse [9], have been associated with the clustered isolates.
To investigate in vivo the impact of TB due to a clustered isolate, we conducted a cohort study of 139 patients treated for drug-susceptible pulmonary TB with the outcomes of survival and sputum sterilization. We hypothesized that patients infected with clustered isolates would have a decreased survival rate and a prolonged time to sputum sterilization, compared with patients infected with nonclustered isolates.
Patients And Methods
Patients. From 1 January 1994 through 30 June 1996, a total of 199 consecutive cases of culture-confirmed TB were reported to the Baltimore City Health Department Tuberculosis Clinic (Maryland). Of the isolates collected during this period, 182 underwent RFLP analysis; 17 additional isolates were unavailable for DNA fingerprinting. Subjects were considered eligible for the study if their isolates were in this RFLP library, which has been described elsewhere [9]. We excluded from this cohort all patients with isolated extrapulmonary TB, because their cases could not be followed by serial culture. In addition, we excluded patients aged <18 years, those with a postmortem diagnosis of TB, and patients who had an M. tuberculosis isolate that was resistant to ⩾1 first-line treatment drugs. The remaining 139 patients were included in the study.
A technician who was blinded to the clinical data performed DNA fingerprinting analysis by use of the standard IS6110 RFLP typing method [13, 14]. Strains that had ⩽6 IS6110 bands also underwent further analysis with a probe specific for the polymorphic GC-rich repetitive sequence [15, 16]. RFLP clusters were composed of ⩾2 isolates with identical IS6110 fingerprints and an identical polymorphic GC-rich repetitive sequence pattern if ⩽6 IS6110 bands were present. A “clustered patient” was defined as a patient who was infected with an isolate included in an RFLP cluster. Patients with an M. tuberculosis RFLP pattern that did not match that of any other isolate were classified as “nonclustered patients.” Because some patients with available DNA fingerprinting results were excluded from the study, the number and specific types of strains within each cluster were not included in the analysis.
Detailed information on patient medical history and TB treatment, including all outpatient and inpatient visits, was abstracted from the Tuberculosis Clinic's medical charts, recorded on a standardized form, and keyed into a computer database; data entry checks were used to ensure accuracy. HIV infection status was determined on the basis of documented results of serologic testing. If results of serologic testing for HIV were not available, then the patient was classified as having high risk or low risk on the basis of drug use and sexual history. An arbitrary threshold of ⩾20 pounds was used as the definition of “weight loss.”
Outcome measures. The outcomes of the study were death due to any cause while the patient was undergoing treatment for TB and conversion of the sputum culture result from being positive for M. tuberculosis to being negative for such organisms. All deaths were confirmed by evaluation of death certificates; however, cause of death was not recorded, because this source of information was considered inconsistent. Survival was measured from the date that treatment began until either the date that death occurred or the date that the last treatment dose was received, at which time patients were censored.
Time to conversion of the sputum culture result was measured from the date that treatment began until the date that the first of 2 consecutive negative sputum culture results was collected. All the patients were followed by Tuberculosis Clinic staff, regardless of whether the patients were treated as outpatients or as inpatients in hospitals or long-term care facilities. Outpatients underwent initial chest radiography; they had their sputum samples cultured on a monthly basis until 2 consecutive negative results were obtained. The sputum samples were processed at the Maryland Department of Health and Mental Hygiene Mycobacteria Laboratory (Baltimore), by use of both the BACTEC radiometric method (Becton Dickinson) and Lowenstein-Jensen slants; they were monitored for 6 weeks before being discarded. Bacteriologic data were abstracted from laboratory reports and recorded on a separate data-collection form, which was identified only by a clinic identification number.
Statistical analysis. Initial analysis was performed with Pearson's χ2 test or Fisher's exact test, for categorical variables, and with a 2-tailed t test or the Mann Whitney U test, for continuous variables. Kaplan-Meier plots, the log-rank test, and Cox proportional hazards modeling were performed to study data on death. Survival data were initially analyzed on an intention-to-treat basis; analysis was then repeated, with days of treatment used as a time-dependent variable. Length of treatment was calculated from the date that treatment began until the date of death or, for censored patients, completion of therapy or loss to follow-up. The proportional hazards assumption was tested and was met with age divided into appropriate categories. Multivariate models, including variables associated with both death and the covariate, were performed to control for potential confounding. Stratified analysis was used to assess for interactions. A 2-tailed α level of 0.05 was used. Statistical analysis was performed with the STATA software package, version 6.0 (Stata).
Results
Patients. Of the original cohort of 182 culture-positive patients for whom DNA fingerprinting results were available, 43 were excluded from the study. Of these 43 patients, 11 received a postmortem diagnosis, 22 had isolated extrapulmonary disease, and 3 were unable to provide sputum samples. Also excluded were 6 patients with M. tuberculosis that was resistant to ⩾1 of the standard treatment drugs and 1 patient aged <18 years.
The demographic and clinical characteristics of the patients are summarized in table 1. Age distribution was normal, and the mean patient age (±SD) was 53 ± 17 years. The majority of the patients were male (100 [72%]) and black (102 [73%]). A total of 31 of the patients (24%) had serologic documentation of HIV infection. Other underlying medical conditions included diabetes (in 18 patients [14%]), renal disease (in 12 [9%]), and chronic obstructive pulmonary disease (COPD; in 13 [10%]). Four patients (3%) had both diabetes and renal disease.
Demographic and clinical characteristics of 139 adult patients with culture-positive pulmonary tuberculosis.
For 73 patients (53%), the M. tuberculosis isolate was classified as clustered by DNA fingerprinting. The remaining 66 patients (47%) had an isolate with a unique RFLP pattern. Patients with clustered isolates were younger than patients with nonclustered isolates (mean age, 47.5 vs 58.2 years; P < .001), and they were more likely to be HIV seropositive (32% vs. 15%; P = .04) and injection drug users (36% vs. 7%; P < .001). Data on race, sex, and underlying medical conditions, including alcohol use, were comparable for patients with clustered and nonclustered isolates.
Approximately half of the patients (73 [53%] of 139) had an initial sputum smear that showed acid-fast bacilli. Tuberculin skin test results were reactive for 71 (68%) of the 104 patients tested. One-third of the patients with nonreactive results had HIV infection. Chest radiography done at the time of diagnosis showed noncavitary disease in 91 patients (65%), cavitary disease in 44 patients (32%), and no abnormality in 4 patients (3%). Cavitary disease was less likely to occur in HIV-seropositive patients than in HIV-seronegative patients (11% vs. 40%; P < .01), but it occurred equally among patients with clustered and nonclustered isolates.
For 129 patients (93%), initial treatment included isoniazid, rifampin, and pyrazinamide. Most of the patients (117 [84%] of 139) received directly observed therapy for their entire course of treatment. Ten of the 22 patients who self-administered therapy did so only before presentation to the clinic (mean duration of self-administration, 11 days; range, 2–28 days). For 40 patients (29%), there was a delay of >14 days between the date that the first positive sputum culture result was obtained and the date that therapy was begun (mean delay, 29 days; range, 15–81 days). Eight patients were lost to follow-up after a mean of 130 days (range, 14–339 days).
Death. A total of 29 (21%) of the 139 patients died during treatment; the median time to death among these patients was 39 days. The median follow-up for survivors was 202 days. Age was strongly associated with the risk of death (table 2). Patients aged >60 years had a 5-fold increased risk of death, compared with patients aged 18–60 years. Patients with clustered DNA fingerprint patterns had a 50% reduced risk of death, compared with patients who had unique patterns (hazard ratio [HR], 0.5; 95% CI, 0.2–1.1). The association was confounded by age, because patients with clustered isolates were significantly younger (age-adjusted HR, 0.8; 95% CI, 0.4–1.8) than patients with nonclustered isolates. Analysis of RFLP clustering according to age strata did not reveal significant evidence for an interaction, although the number of deaths among older patients with clustered isolates was small. When HIV infection and injection drug use, factors associated with RFLP clustering, were entered along with age into the Cox model, the results remained unchanged. Patients with positive sputum smear results and patients with cavitary disease did not have an increased risk of dying. Patients with a reactive tuberculin skin test result had a 77% reduced risk of death (HR, 0.23; 95% CI, 0.1–0.6), independent of age, HIV infection, and injection drug use. A delay of >14 days before initiation of therapy was not associated with increased mortality.
Molecular epidemiologic, clinical, and demographic characteristics and risk of death due to all causes for 139 patients treated for culture-positive tuberculosis.
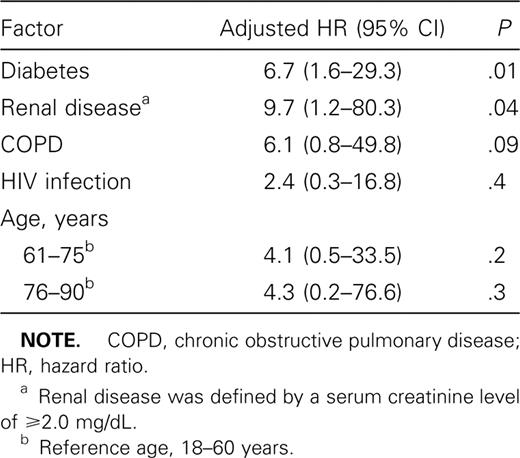
Diabetes, renal disease, COPD, and HIV infection were independent predictors of death, after adjustment for age (figure 1 and table 2). Renal disease represented the greatest increased risk of death, with 9 (75%) of the 12 patients with renal disease dying while receiving treatment (HR, 9.4; 95% CI, 4.1–21.8). Univariate analysis of data on steroid use showed an increased risk of death among steroid users with TB. However, 4 of 7 patients who were using steroids also had a history of diabetes, and 1 had COPD. When results were adjusted for the presence of diabetes and COPD, steroid use was no longer a significant predictor of death. A history of cancer (8 patients) was associated with a trend toward an increased risk of death (HR, 2.5; 95% CI, 0.7–8.5). When the diseases that were significantly associated with death in the univariate analysis were analyzed in a multivariate model, only diabetes and renal disease remained independent predictors of death (table 3). Demographic variables, including race, sex, and homelessness, were not associated with death. Self-reported daily alcohol consumption also was not associated with death. For injection drug users, the age-adjusted risk of death was no longer significant when controlled for HIV infection (HR, 5.5; 95% CI, 0.5–54). A separate Cox regression model, in which treatment was used as a time-dependent variable, found associations that were similar in magnitude and significance to those revealed by the intention-to-treat model.
Kaplan-Meier survival curves for patients with culture-positive pulmonary tuberculosis, by underlying illnesses (HIV infection, diabetes, renal failure, or chronic obstructive pulmonary disease [COPD]). Renal failure is defined by a serum creatinine level of ⩾2.0 mg/dL.
Multivariate Cox proportional hazards model for mortality, including diseases associated with mortality in univariate analysis.
Conversion of the sputum culture result. Because 27 (90%) of 30 patients whose sputum culture result did not convert from positive to negative died during follow-up, significantly fewer culture samples were obtained from these patients (mean number of samples, 3 vs. 9). To minimize information bias, we analyzed the median time to conversion of the sputum culture result for those patients who had sputum sterilization occur (109 of 139 patients), instead of using conversion of the sputum culture result as an outcome. Time to sterilization was set at 0 days for the 3 patients who had negative sputum culture results before initiation of therapy. The median time to sputum sterilization was 59 days (range, 0–458 days). Time to sterilization was prolonged for patients with smear-positive sputum samples or cavitary lung disease (table 4). Patients who had a delay of >14 days between the date that the first positive culture result was obtained and the date that treatment was initiated had shorter times to sputum conversion than did patients with little or no delay in receiving treatment (median number of days, 45 vs. 68; P < .001). The delay in treatment initiation did not affect the number of sputum samples collected (mean number of samples, 7.2 vs. 8.1). With regard to time to conversion of the sputum culture result, there was no difference between patients with RFLP-clustered isolates and those with nonclustered isolates (P = .5). Underlying medical illnesses and demographic variables did not affect the time to sputum sterilization. HIV-infected patients tended to have a shorter time to sterilization than did HIV-negative patients (median days, 44 vs. 62, P = .06).
Median duration of therapy until conversion of the sputum culture result for 109 patients, according to intention-to-treat analysis.
Discussion
In this community-based cohort of patients with drug-susceptible pulmonary TB, we found that older age, diabetes, renal disease, COPD, and HIV infection were associated with an increased risk of death during treatment. Patients infected with RFLP clustered strains tended to be less likely to die but were younger than patients infected with unique strains. Intact cellular immune response, which was indicated by a reactive tuberculin skin test result, was associated with a 77% reduced risk of death.
Death remains a common outcome for patients with TB. Case-fatality rates are reported to be between 7% and 35% [17–19]. Delayed treatment and infection with multidrug-resistant strains of mycobacteria are associated with increased risk of death [20–22]. HIV-infected patients have a 4–8-fold increased risk of dying with TB, compared with HIV-seronegative patients [20, 23–25].
The effect of other underlying diseases on the risk of death due to TB has not been studied as extensively as the effect of HIV infection. On death certificates from 1990, the most common diseases that were listed with TB as the cause of death included AIDS (11%), cardiovascular disease (16%), cancer (9%), and COPD (5%) [26]. A case-control study of 50 patients with TB showed that 56% of the patients who died had ⩾1 clinical risk factor, compared with 14% of the control group (i.e., hospitalized survivors) [27]. Before the HIV pandemic, investigators in British Columbia found that chronic renal disease, malignancy, and silicosis were associated with the deaths of 153 patients treated for TB [28]. A recent study of death among hospitalized patients following diagnosis of TB found that respiratory failure requiring mechanical ventilation, malnutrition, and end-stage renal disease were predictors of death [29]. Our study extends these findings to a community-based population that represents patients with TB from an entire city, with consistent treatment and follow-up.
Questions remain regarding the pathogenesis of fatal TB. Cell-mediated immunity is the key to defense against M. tuberculosis [30, 31]. Among HIV-infected patients, a positive delayed-type hypersensitivity reaction to tuberculin (a marker for intact cellular immunity) has been associated with a reduced risk of death, compared with that noted for anergic patients [32, 33]. Although renal disease has been recognized as a risk factor for reactivation of latent disease, its effect on mortality may be caused by a combination of altered cellular immunity [34], difficulty of the diagnosis [35], or death resulting from other causes. Association of diabetes and COPD with deaths due to TB may have a similar basis. The significant and independent effect of diabetes and renal disease on mortality emphasizes the importance of host factors in determining the outcome of TB. Although there are data suggesting that some M. tuberculosis strains may be highly efficient in establishing initial infection [2, 11], we found that patients infected with clustered strains had clinical outcomes similar to those of patients infected with unique strains. Our results suggest that host factors are more important than strain variation in determining the outcome of pulmonary TB.
We found no association between alcohol abuse and survival, although such an association has been reported elsewhere [20, 36]. The effect of alcohol abuse on patient risk of death may have been diluted by our study's broad definition of alcohol abuse, which included daily consumption of any quantity of alcohol. In addition, because 91% of our patients received directly observed therapy for the majority of their chemotherapy, the effect of poor adherence associated with alcohol may have been minimized.
As an additional outcome, we studied the length of time required to render M. tuberculosis nonviable in sputum samples. We found that a prolonged time to conversion of the sputum culture result was associated with cavitary lung disease and the presence of acid-fast bacilli in the initial smear sample. It is unclear whether these results can be attributed to increased pathogenicity of the organism, apart from burden or altered host response. Neither the RFLP cluster status of the isolate nor host factors affected clearance of the organism. In fact, HIV-infected patients tended to sterilize their sputum more quickly than did seronegative patients. This finding is likely due to the low number of HIV-infected patients with cavitary disease (11% vs. 40%), but it may explain why some HIV-infected patients have been reported to be less infectious to contacts [37, 38].
The apparent accelerated sputum sterilization for patients experiencing a delay in treatment is likely because of diagnostic bias. On the basis of their clinical presentation, these patients were not deemed sick enough to receive empiric chemotherapy, but they were closely followed and did well once therapy was begun. Delayed treatment of TB can have serious consequences and has been associated with increases in the mortality rate in other studies [20, 21]. These findings do not minimize the importance of prompt diagnosis and treatment of TB; rather, they suggest that patients who have atypical chest radiography findings and smears that are negative for acid-fast bacilli will have more rapid sterilization of their sputum.
A limitation of the present study is its exclusion of patients who had cultures that were negative for TB and those whose disease was entirely extrapulmonary. Our results may not apply to these patients, who may have a different clinical presentation and prognosis. Our selection of patients may explain the apparent high overall mortality rate in the present study (21%), compared with the 16% case-fatality rate reported in the Americas in 1999 [17]. The conclusions made on the basis of RFLP clustering are dependent on appropriate designation of clustered status versus nonclustered status. Although our subjects were selected from a cohort of patients whose isolates were in an RFLP library that included 91% of the culture-positive isolates in Baltimore City during the study period, misclassification could have occurred. However, other published data from this cohort show that clustered cases of TB are grouped according to geographic areas [9]. This finding suggests that recently transmitted TB isolates should be equally accessible for DNA fingerprinting.
We conclude that comorbidity has a significant affect on the survival of patients with pulmonary TB and that it is a poor prognostic indicator that must be considered regardless of sputum conversion. The results of the present study highlight the role of M. tuberculosis as an opportunistic infection in patients with and without HIV infection; they also underscore the importance of prompt diagnosis of infection and use of preventive therapy for individuals with chronic diseases. The finding that infection with genetically clustered M. tuberculosis strains appears to have no affect on either sputum sterilization or mortality suggests that transmissible strains of M. tuberculosis are not associated with increased virulence.
acknowledgments
We wish to thank the staff at the Baltimore City Health Department Tuberculosis Clinic for their excellent clinical care and thorough follow-up.
references
Financial support: National Institutes of Health (grants T32A107201, AI01637, AI36973, DA07061, and AI40605) and Centers for Disease Control and Prevention Cooperative Agreement (grant U300466-10).
Present affiliation: University of Maryland School of Medicine, Baltimore.
- diabetes mellitus
- lung diseases
- kidney failure, chronic
- pulmonary tuberculosis
- adult
- comorbidity
- dna
- dna fingerprinting
- epidemiologic factors
- restriction fragment length polymorphism
- tuberculosis
- therapeutic immunosuppression
- natural immunosuppression
- infections
- mortality
- hiv infections
- fingerprints




![Kaplan-Meier survival curves for patients with culture-positive pulmonary tuberculosis, by underlying illnesses (HIV infection, diabetes, renal failure, or chronic obstructive pulmonary disease [COPD]). Renal failure is defined by a serum creatinine level of ⩾2.0 mg/dL.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/34/6/10.1086_338784/1/m_34-6-752-fig001.jpeg?Expires=1716329258&Signature=z07yWrhPNFO2xPyuiyiScuyzdQVAdCIiGXkySNBw~q90b4iJQBlHxdaPLu-ka~cJ6KZ2zIeweyrvdjQ0OP7FxKa7E74eH4SBOgXTqF3gb3BX3i8KWqHK23zqwqnZ1fG4xf~wAM1YZGd3TvNUM2Qam99mYNfbEnmloIYm7V9etcim4kJ3CE~8-BwMnKTV1KHHOI~oIsdOTxjdGtqjsTCkvSDShWz7d6OksMy00ogumLuKqJQ-jbbbbbFd4ytso4JAscBdPbVHa7i1b2fWWY5cdjrkwO2VBQ39o~GRq3Mn-Uco0V8bY6Qtl2E7INCae0KBZ4Dxkh16RHjkoAykgU1MZw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments