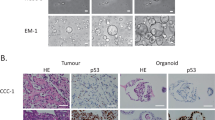
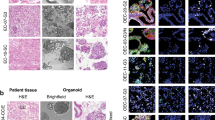
Abstract
Ovarian cancer (OC) is a heterogeneous disease usually diagnosed at a late stage. Experimental in vitro models that faithfully capture the hallmarks and tumor heterogeneity of OC are limited and hard to establish. We present a protocol that enables efficient derivation and long-term expansion of OC organoids. Utilizing this protocol, we have established 56 organoid lines from 32 patients, representing all main subtypes of OC. OC organoids recapitulate histological and genomic features of the pertinent lesion from which they were derived, illustrating intra- and interpatient heterogeneity, and can be genetically modified. We show that OC organoids can be used for drug-screening assays and capture different tumor subtype responses to the gold standard platinum-based chemotherapy, including acquisition of chemoresistance in recurrent disease. Finally, OC organoids can be xenografted, enabling in vivo drug-sensitivity assays. Taken together, this demonstrates their potential application for research and personalized medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
BAM files for DNA and RNA sequencing data are made available through controlled access at the European Genome-phenome Archive (EGA) which is hosted at the EBI and the CRG (https://ega-archive.org), under accession number EGA: EGAS00001003073. Data access requests will be evaluated by the UMCU Department of Genetics Data Access Board (EGAC00001000432) and transferred on completion of a material transfer agreement and authorization by the medical ethical committee UMCU at request of the HUB to ensure compliance with the Dutch ‘medical research involving human subjects’ act.
Code availability
Illumina data processing pipeline v2.2.1 is available at https://github.com/UMCUGenetics/IAP/releases/tag/v2.2.1 and RNA analysis pipeline v2.3.0 is available at https://github.com/UMCUGenetics/RNASeq. All other custom code used for this study is available at https://github.com/UMCUGenetics/OvCaBiobank
References
Vaughan, S. et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat. Rev. Cancer 11, 719–725 (2011).
Bowtell, D. D. et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 15, 668–679 (2015).
Fischerova, D. et al. Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist 17, 1515–1533 (2012).
Koshiyama, M., Matsumura, N. & Konishi, I. Recent concepts of ovarian carcinogenesis: type I and type II. Biomed. Res. Int. 2014, 934261 (2014).
Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
Ciriello, G. et al. Emerging landscape of oncogenic signatures across human cancers. Nat. Genet. 45, 1127–1133 (2013).
Piek, J. M. et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J. Pathol. 195, 451–456 (2001).
Kurman, R. J. & Shih Ie, M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am. J. Surg. Pathol. 34, 433–443 (2010).
Thu, K. L. et al. A comprehensively characterized cell line panel highly representative of clinical ovarian high-grade serous carcinomas. Oncotarget 8, 50489–50499 (2017).
Fleury, H. et al. Novel high-grade serous epithelial ovarian cancer cell lines that reflect the molecular diversity of both the sporadic and hereditary disease. Genes Cancer 6, 378–398 (2015).
Ince, T. A. et al. Characterization of twenty-five ovarian tumour cell lines that phenocopy primary tumours. Nat. Commun. 6, 7419 (2015).
Letourneau, I. J. et al. Derivation and characterization of matched cell lines from primary and recurrent serous ovarian cancer. BMC Cancer 12, 379 (2012).
Kreuzinger, C. et al. Molecular characterization of 7 new established cell lines from high grade serous ovarian cancer. Cancer Lett. 362, 218–228 (2015).
Sachs, N. & Clevers, H. Organoid cultures for the analysis of cancer phenotypes. Curr. Opin. Genet. Dev. 24, 68–73 (2014).
Domcke, S. et al. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 4, 2126 (2013).
Jones, P. M. & Drapkin, R. Modeling high-grade serous carcinoma: how converging insights into pathogenesis and genetics are driving better experimental platforms. Front. Oncol. 3, 217 (2013).
Ben-David, U. et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 49, 1567–1575 (2017).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Fujii, M. et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18, 827–838 (2016).
Boj, S. F. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338 (2015).
van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Roerink, S. F. et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 556, 457–462 (2018).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373–386.e10 (2018).
Verissimo, C. S. et al. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. Elife 5, e18489 (2016).
Hill, S. J. et al. Prediction of DNA repair inhibitor response in short term patient-derived ovarian cancer organoids. Cancer Discov. 8, 1404–1421 (2018).
Kessler, M. et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 6, 8989 (2015).
Gilmour, L. M. et al. Neuregulin expression, function, and signaling in human ovarian cancer cells. Clin. Cancer Res. 8, 3933–3942 (2002).
Aune, G. et al. Increased circulating hepatocyte growth factor (HGF): a marker of epithelial ovarian cancer and an indicator of poor prognosis. Gynecol. Oncol. 121, 402–406 (2011).
Sheng, Q. et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell 17, 298–310 (2010).
Bourgeois, D. L. et al. High-grade serous ovarian cancer cell lines exhibit heterogeneous responses to growth factor stimulation. Cancer Cell Int. 15, 112 (2015).
Antoniou, A. et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am. J. Hum. Genet. 72, 1117–1130 (2003).
Gabai-Kapara, E. et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc. Natl Acad. Sci. USA 111, 14205–14210 (2014).
Wang, M. et al. PAX2 and PAX8 reliably distinguishes ovarian serous tumors from mucinous tumors. Appl. Immunohistochem. Mol. Morphol. 23, 280–287 (2015).
Rajagopalan, H. & Lengauer, C. Aneuploidy and cancer. Nature 432, 338–341 (2004).
Priestley, P. et al. Pan-cancer whole genome analyses of metastatic solid tumors. Preprint at https://doi.org/10.1101/415133 (2018).
Gorringe, K. L. et al. High-resolution single nucleotide polymorphism array analysis of epithelial ovarian cancer reveals numerous microdeletions and amplifications. Clin. Cancer Res. 13, 4731–4739 (2007).
Hunter, S. M. et al. Pre-invasive ovarian mucinous tumors are characterized by CDKN2A and RAS pathway aberrations. Clin. Cancer Res. 18, 5267–5277 (2012).
Romero, I. et al. Low-grade serous carcinoma: new concepts and emerging therapies. Gynecol. Oncol. 130, 660–666 (2013).
Kuo, K. T. et al. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res. 69, 4036–4042 (2009).
Seidman, J. D. et al. The fallopian tube–peritoneal junction: a potential site of carcinogenesis. Int. J. Gynecol. Pathol. 30, 4–11 (2011).
Kurman, R. J. & Shih Ie, M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer–shifting the paradigm. Hum. Pathol. 42, 918–931 (2011).
Seidman, J. D. & Khedmati, F. Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) neoplasms and their relationship with Walthard cell nests: a study of 120 tumors. Arch. Pathol. Lab. Med. 132, 1753–1760 (2008).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Yadav, B. et al. Quantitative scoring of differential drug sensitivity for individually optimized anticancer therapies. Sci. Rep. 4, 5193 (2014).
Lord, C. J. & Ashworth, A. PARP inhibitors: synthetic lethality in the clinic. Science 355, 1152–1158 (2017).
Murai, J. Targeting DNA repair and replication stress in the treatment of ovarian cancer. Int. J. Clin. Oncol. 22, 619–628 (2017).
Meijer, T. G. et al. Functional ex vivo assay reveals homologous recombination deficiency in breast cancer beyond BRCA gene defects. Clin. Cancer Res. 24, 6277–6287 (2018).
Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521, 43–47 (2015).
Matano, M. et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 21, 256–262 (2015).
Drost, J. et al. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science 358, 234–238 (2017).
Fumagalli, A. et al. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc. Natl Acad. Sci. USA 114, E2357–E2364 (2017).
Schmeler, K. M. et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol. Oncol. 108, 510–514 (2008).
Gershenson, D. M. et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol. Oncol. 114, 48–52 (2009).
Pectasides, D. et al. Advanced stage mucinous epithelial ovarian cancer: the Hellenic Cooperative Oncology Group experience. Gynecol. Oncol. 97, 436–441 (2005).
Brown, J. & Frumovitz, M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr. Oncol. Rep. 16, 389 (2014).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM.Preprint at https://arxiv.org/abs/1303.3997 (2013).
Van der Auwera, G. A. et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics 43, 11.10.1–11.10.33 (2013).
McKenna, A. et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Saunders, C. T. et al. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 28, 1811–1817 (2012).
Koboldt, D. C. et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 22, 568–576 (2012).
Garrison, E. & Marth, G. Haplotype-based variant detection from short-read sequencing. Preprint at https://arxiv.org/abs/1207.3907 (2012).
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 31, 213–219 (2013).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6, 80–92 (2012).
Boeva, V. et al. Control-FREEC: a tool for assessing copy number and allelic content using next-generation sequencing data. Bioinformatics 28, 423–425 (2012).
Chen, X. et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016).
The Genome of the Netherlands Consortium. Whole-genome sequence variation, population structure and demographic history of the Dutch population. Nat. Genet. 46, 818–825 (2014).
Genomes Project, C. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Muraro, M. J. et al. A single-cell transcriptome atlas of the human pancreas. Cell Syst. 3, 385–394.e3 (2016).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 8, 1754–1760 (2009).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Durinck, S. et al. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 4, 1184–1191 (2009).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Aryee, M. J. et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30, 1363–1369 (2014).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Koo, B. K. et al. Controlled gene expression in primary Lgr5 organoid cultures. Nat. Methods 9, 81–83 (2011).
Acknowledgements
We thank T. Bayram for supporting of ethical regulatory affairs. We acknowledge A. Brousali, P. van der Groep, A. Constantinides, A. Snelting and O. Kranenburg of the Utrecht Platform for Organoid Technology (U-PORT; UMC Utrecht) for patient inclusion and tissue acquisition. We thank the Integraal Kankercentrum Nederland (IKNL) and M. van der Aa for supplying clinical data, and I. Renkens for help with DNA isolations. We acknowledge E. Stelloo for her help with culturing organoids. We thank the people from the Preclinical Intervention Unit of the Mouse Clinic for Cancer and Ageing (MCCA) at the NKI for performing the intervention studies. We thank B. Artegiani and T. Dayton for critically reading the manuscript. O.K. was supported by Marie Skłodowska-Curie IF grant 658933 – HGSOC. This work was funded by the gravitation program CancerGenomiCs.nl from the Netherlands Organisation for Scientific Research (NWO), MKMD grant (114021012) from Netherlands Organization for Scientific Research (NWO-ZonMw), Stand Up to Cancer International Translational Cancer Research Grant, a program of the Entertainment Industry Foundation administered by the AACR, Dutch Cancer Society (KWF) grant UU2015-7743, Dutch Cancer Society (Alpe d’HuZes) grant EMCR 2014-7048, and a grant from the Gieskes Strijbis Foundation (1816199). The Oncode Institute is supported by the Dutch Cancer Society.
Author information
Authors and Affiliations
Contributions
Conceptualization: O.K. and H.C. Methodology: O.K. and H.C. Software: J.E.V.-I., M.J.v.R., L.K. and W.P.K. Formal analysis: O.K., K.L., N.H., J.E.V.-I., M.J.v.R., T.J., P.J.V.D., S.A.R., L.K. and W.P.K. Investigation: O.K., K.L., C.J.d.W., N.H., A.V.B., H.B., J.K., S.A.R., L.K., N.P., R.T., L.M.v.W. and B.P. Resources: C.J.d.W., L.M.v.W., H.V., M.P.G.V., V.W.H.H., B.G.N., P.O.W., M.V.D.V., T.B, K.N.G. and R.P.Z. Data curation: O.K., C.J.d.W. and J.E.V.-I. Writing—original draft: O.K., C.J.d.W., J.E.V.-I., W.P.K. and H.C. Visualization: O.K., C.J.d.W., K.L., J.E.V.-I., W.P.K., L.K. and N.H. Supervision: M.V.D.V., J.L.B., R.P.Z., H.J.G.S., W.P.K., A.v.O. and H.C. Project administration: O.K., C.J.d.W., W.P.K. and H.C. Funding acquisition: J.L.B., R.P.Z., P.O.W., W.P.K. and H.C.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Derivation and morphological differences of OC organoids.
a, Schematic of OC organoid derivation. b, Bright-field images of MBT, SBT, MC, LGS, END and CCC organoids (left to right), depicting different organoid morphologies. Morphological description of 50 independent organoid lines is provided in Supplementary Table 6. Scale bar, 100 μm. c, Bright-field (top) and SEM (bottom) images demonstrating main morphologies among different HGS organoid lines. Starting with cystic and well-organized cellular polarity, where microvilli are directed toward the organoid lumen (most left) to dense organoids that gradually (from left to right) show reduced circularity and cellular cohesiveness up to a grape-like shape morphology (most right). Scale bar, 100 μm. d, High-magnification H&E staining images displaying representative examples of HGS organoid morphologies as well as nuclear and cellular atypia, typically displayed by HGS tumors. Histological description of 50 independent organoid lines is provided in Supplementary Table 6. Scale bar, 100 μm.
Extended Data Fig. 2 Organoid passage number overview and normal cell contamination in tumors and organoids.
a, Column bar graph depicting organoid maximum passage number up until the moment of submission. Organoids that stopped/slowed down their growth are indicated in orange. b, Representative images of Ki67 staining of six independent organoid lines show a high percentage of ki67-positive proliferating cells. c, Histological and immunohistochemical images of tumor tissue (derived from two independent patients) showing tumor cell purity within different samples, based on H&E and p53 staining. Scale bar, 0.5 mm. d, Tukey box-and-whisker plot (1.5× interquartile range) presenting bioinformatic estimation of tumor cell purity percentage of both tissue (n = 35) and organoid (n = 36) based on WGS data using PURPLE. Horizontal bars represent median of all dots. Mean and standard deviation across all samples are as follows: 45 ± 9.2% (tissue) and 88.1 ± 23% (organoids). e, Stacked bar chart showing the percentage of organoid lines that are positive for p53, PAX8 and periodic acid–Schiff (PAS) staining (orange) and negative (blue) grouped per original tumor staining status (see also Supplementary Table 6). Total number (n) of tissues stained per group are indicated.
Extended Data Fig. 3 FT and OSE organoids.
a, An overview image of normal FT organoids embedded in 40 μl BME drops, displaying a cystic morphology. All FT organoid lines that were established (n = 22) displayed cystic morphology. b, Representative SEM image showing ciliated cells facing FT organoid lumen. Scale bar, 50 μm. SEM was performed on one FT organoid line. c, Histological analysis of FT organoids demonstrating H&E, Ki67, PAX8 and Ac-α-tubb staining. Histological analysis was performed on three independent FT organoid lines with similar results. Scale bar, 100 μm. d, An overview image of normal OSE organoids embedded in 40 μl BME drops displaying cystic morphology (top left image). Seven out of eight OSE organoid lines that were established displayed cystic morphology. OSE organoid images of H&E, Ki67 and cytokeratin 8 (CK8) staining, demonstrating a cystic morphology of proliferative epithelial cells. Histological analysis was performed on two independent OSE organoid lines with similar results. Scale bar, 100 μm. e, First row: bright-field images of LGS-2.2 (left) and OSE(P)7 (right) organoid lines. Unlike normal FT and OSE that display cystic morphology both lines show dense phenotype. OSE(P)7 is the only OSE organoid line that display dense phenotype. Scale bar, 200 μm. Second to last rows: histological and immunohistochemical images demonstrate that organoids are positively stained for PAX8 and WT1, markers of OC serous subtypes. Organoids display reduced cellular organization in comparison to normal FT and OSE organoids. Scale bar, 100 μm. f, Scatter plot presenting metaphase spread analysis and mean for each line. Both lines present aneuploidy.
Extended Data Fig. 4 Genome-wide tumor and organoid pair comparison.
a, Genome-wide CNVs in tumor/organoid pairs (black, tumors; pink, organoids early passage; blue, organoids late passage) depicting gains (red) and losses (blue). b, Number of shared (yellow) and unique (blue) SNVs (on the left) and SVs (on the right) between tumor/organoid pairs. Shared variants are those that can be found in the corresponding paired sample. Passage number at which organoid lines were sequenced is given in Supplementary Table 7.
Extended Data Fig. 5 Molecular characterization, drug screening and xenografts of OC organoids.
a, Tukey box and whisker plot (1.5× interquartile range) summarizing the percentage of shared variants across all tumor (red) and organoid (green) samples. Right and left panels display SNVs and SVs, respectively. Horizontal bars represent median of all dots. Mean and standard deviation across all samples are as follows: SNVs, 82.95 ± 8.18% (tissue, n = 31) and 75.62 ± 23.13% (organoids, n = 31); SVs, 78.14 ± 22.11% (tissue, n = 31) and 60.47 ± 29.13% (organoids, n = 31). Samples with a low percentage of shared variants are indicated. b, Heat map of five independent organoid lines from both early and late passages based on 11,720 methylation probes. The heat map colors represent Pearson correlation values, as calculated from the methylation beta-values. Clustering of the correlation values was performed using hierarchical clustering based on complete linkage. c, Scatter plot of AUC values across all drug screening data, displaying high correlation between technical replicates (Pearson correlation = 0.94, R2 = 0.88, n = 105). d, Scatter plot of AUC values of biological replicates, displaying high correlation (Pearson correlation = 0.87, R2 = 0.74, n = 45). Colored dots represent biological replicates in which passage differences between experimental repetition is as follows: 1–2 passages, n = 29 (black); 3–5 passages, n = 10 (blue) and 13–22 passages, n = 6, (red), demonstrating stable drug sensitivity even after prolonged passaging. e, Box-and-whisker plot (10th–90th percentile) showing Z-factor distribution and mean across all drug screening plates. Mean = 0.61, ranging between 0.2 and 0.91, n = 55. f, Bioluminescence imaging of mice, orthotopically transplanted with luciferase expressing organoid lines depicting tumor growth. A summary of organoid-derived xenograft experiments is presented in Supplementary Table 8. g, p53 staining of organoid-derived xenograft (HGS-3.1) on orthotopic transplantation into the mouse bursa shows p53 overexpression in tumor cells. h, Histological analysis of an organoid-derived xenograft (MC-2.1) on subcutaneous transplantation. H&E staining shows haphazardly arranged neoplastic glands lined by columnar cells with variable numbers of goblet cells (arrows), which are specific features of MC. A summary of organoid-derived xenograft experiments is presented in Supplementary Table 8. Left image scale bar, 1 mm. Right image scale bar, 200 μm.
Extended Data Fig. 6 CRISPR–Cas9 mediated genetic manipulation in FT organoids.
a, Schematic of normal FT organoid electroporation. FT organoids were dissociated into small cell clumps and electroporated with either an empty vector or a vector containing a gRNA directed against TP53. Cells were plated and after 2 d of recovery nutlin3a was added. b, Overview images of organoids 2 weeks after electroporation. Organoids that were electroporated with an empty vector and not treated with nutlin3a showed nice recovery following electroporation (top), whereas the growth of organoids electroporated in a similar manner was dramatically inhibited when nutlin3a was added (middle). Surviving clones that are not inhibited by nutlin3a treatment are visible only when organoids were electroporated with a vector containing TP53 gRNA (bottom). Four independent electroporation experiments followed by nutlin3A treatment were conducted giving rise to multiple Nutlin3A resistant clones. c, A representative flow cytometry analysis of organoids 48 h following electroporation demonstrating 25% of the cell express GFP. Summary of six independent repetitions of this experiment are presented in d. d, Box-and-whisker plot (minimum to maximum) showing the percentage of GFP positive cells following electroporation. Horizontal bars and dashed horizonal bars represent median and mean of all dots, respectively. Mean ± s.d. = 23.8 ± 5.5%, median = 25.5%. Six independent experiments that were conducted with three different FT organoid lines are presented, demonstrating high and robust electroporation efficiency. e, An example of CRISPR–Cas9 mediated editing of TP53 gene in FT organoids. Targeted locus is presented and gRNA (solid line), PAM sequence (red highlight) and cut point (arrow head) are indicated. Sequencing results revealed out-of-frame deletions induced by CRISPR–Cas9 editing. f, Table presenting six FT genetically engineered clones derived from two independent donors (FT(P)1 and FT(P)2). For each clone, targeted gene description (in both TP53 and RB1 genes) including HGVS nomenclature is presented. (HET, heterozygous; HOM, homozygous). g, BF images (top) and H&E staining (bottom) of four independent clones show deviation from cystic and well-organized normal FT organoid morphology. Passage number is indicated. This analysis was conducted on three independent TP clones (loss-of-function mutations in the TP53 gene) and three independent TPR clones (loss-of-function mutations in the TP53 and RB1 genes) with similar results. h, Heat map of Spearman correlation values of three independent normal FT organoid lines (derived from different donors) and genetically engineered clones (n = 3 independent TP clones (loss-of-function mutations in the TP53 gene) and 3 independent TPR clones (loss-of-function mutations in the TP53 and RB1 genes)), using RNA-seq expression data. Read counts were normalized for sequencing depth and the 1,000 most-variable genes were used. Clones were assigned into different groups according to their mutational profile.
Supplementary information
Supplementary Tables
Supplementary Tables 2, 4 and 8
Supplementary Tables
Supplementary Tables 1, 3, 5–7 and 9–11
Supplementary Video 1
High speed time-lapse imaging of normal FT line with beating cilia. High magnification of FT organoid epithelial cell layer and lumen. Beating ciliated cells are directed into the organoid lumen. High speed time-lapse imaging was conducted on one FT organoid line.
Supplementary Video 2
Live cell imaging of chromosomal segregation in OC organoids (HGS-3.2). Four independent organoid lines were subjected to confocal imaging for 11 h with 4 min intervals. All demonstrated normal and abnormal chromosomal segregations. Shown is a representative time-lapse imaging of HGS-3.2 line. Upper right panel shows H2B-Neon fluorescence after maximum-projection of three-dimensional z-stacks; upper left panel displays the same data, color coded for depth (z), facilitating tracking of individual events; lower panels consist of transmitted light images with and without merged H2B-Neon (green). Scale bar, 20 μm
Supplementary Video 3
Live cell imaging of chromosomal segregation in OC organoids (HGS-3.2). Example of normal chromosomal segregation. Color coded for depth (z).
Supplementary Video 4
Live cell imaging of chromosomal segregation in OC organoids (HGS-3.2). Example of chromosomal segregation into three poles and lagging chromosomes. Color coded for depth (z).
Supplementary Video 5
Live cell imaging of chromosomal segregation in OC organoids (HGS-3.2). Example of chromatin bridge during chromosomal segregation. Color coded for depth (z).
Supplementary Video 6
Live cell imaging of chromosomal segregation in OC organoids (HGS-3.2). Example of mitotic catastrophe that follows chromosomal segregation into three poles. Color coded for depth (z).
Rights and permissions
About this article
Cite this article
Kopper, O., de Witte, C.J., Lõhmussaar, K. et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med 25, 838–849 (2019). https://doi.org/10.1038/s41591-019-0422-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-019-0422-6
This article is cited by
-
CircRAD23B promotes proliferation and carboplatin resistance in ovarian cancer cell lines and organoids
Cancer Cell International (2024)
-
Comprehensive machine learning-based preoperative blood features predict the prognosis for ovarian cancer
BMC Cancer (2024)
-
Decoding the basis of histological variation in human cancer
Nature Reviews Cancer (2024)
-
Establishment and characterization of multiple patient-derived organoids from a case of advanced endometrial cancer
Human Cell (2024)
-
Advances and Applications of Cancer Organoids in Drug Screening and Personalized Medicine
Stem Cell Reviews and Reports (2024)