Key Points
-
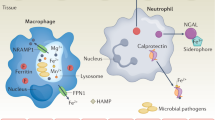
Microbial pathogens require nutrient metals in order to grow and cause disease. However, excess metals are toxic, so metal levels must be tightly regulated during infection. Vertebrates have evolved to exploit this metal dependence and metal toxicity through strategies that either prevent access to nutrient metal or direct excess metals towards invading pathogens. Collectively, these processes are known as nutritional immunity.
-
The struggle between host and pathogen for nutrient metals is best studied in the area of Fe. Fe is sequestered from invading pathogens either intracellularly or in high-affinity Fe-binding proteins. To combat host-mediated Fe sequestration, microbial pathogens elaborate several high-affinity Fe acquisition systems.
-
Recently, vertebrate proteins of the innate immune system have been identified that prevent microbial infection through the chelation of nutrient Mn and Zn. These proteins are members of the S100 family of Ca-binding proteins and are abundant at sites of inflammation. In addition to Mn and Zn sequestration, vertebrates can use strategies to direct toxic levels of Mn and Zn towards microbial pathogens. Bacterial measures to combat Mn and Zn sequestration, as well as the toxicity that is associated with excess levels of these metals, are beginning to be uncovered.
-
It is becoming increasingly evident that host-mediated direction of excess Cu towards microbial pathogens is a crucial aspect of vertebrate defence against infection. This observation has provided an explanation for the broad conservation of Cu detoxification systems across disease-causing microorganisms.
-
The importance of nutritional immunity for defence against infection is highlighted by the observation that inherited defects in transition metal homeostasis dramatically affect host susceptibility to certain infectious diseases. This fact underscores the tremendous therapeutic potential of targeting bacterial metal acquisition systems.
Abstract
Transition metals occupy an essential niche in biological systems. Their electrostatic properties stabilize substrates or reaction intermediates in the active sites of enzymes, and their heightened reactivity is harnessed for catalysis. However, this heightened activity also renders transition metals toxic at high concentrations. Bacteria, like all living organisms, must regulate their intracellular levels of these elements to satisfy their physiological needs while avoiding harm. It is therefore not surprising that the host capitalizes on both the essentiality and toxicity of transition metals to defend against bacterial invaders. This Review discusses established and emerging paradigms in nutrient metal homeostasis at the pathogen–host interface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Andreini, C., Bertini, I., Cavallaro, G., Holliday, G. L. & Thornton, J. M. Metal ions in biological catalysis: from enzyme databases to general principles. J. Biol. Inorg. Chem. 13, 1205–1218 (2008).
Andreini, C., Bertini, I. & Rosato, A. Metalloproteomes: a bioinformatic approach. Acc. Chem. Res. 42, 1471–1479 (2009).
Andreini, C., Banci, L., Bertini, I. & Rosato, A. Zinc through the three domains of life. J. Proteome Res. 5, 3173–3178 (2006).
Weinberg, E. D. Nutritional immunity. Host's attempt to withold iron from microbial invaders. JAMA 231, 39–41 (1975).
Weinberg, E. D. Iron availability and infection. Biochim. Biophys. Acta 1790, 600–605 (2009).
Cassat, J. E. & Skaar, E. P. Metal ion acquisition in Staphylococcus aureus: overcoming nutritional immunity. Semin. Immunopathol. 34, 215–235 (2011).
Haley, K. P. & Skaar, E. P. A battle for iron: host sequestration and Staphylococcus aureus acquisition. Microbes Infect. 14, 217–227 (2011).
Nobles, C. L. & Maresso, A. W. The theft of host heme by Gram-positive pathogenic bacteria. Metallomics 3, 788–796 (2011).
Ong, S. T., Shan Ho, J. Z., Ho, B. & Ding, J. L. Iron-withholding strategy in innate immunity. Immunobiology 211, 295–314 (2006).
Braun, V. & Hantke, K. Recent insights into iron import by bacteria. Curr. Opin. Chem. Biol. 15, 328–334 (2011).
Jabado, N. et al. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192, 1237–1248 (2000). The finding that NRAMP1 protects against infection by extrusion of divalent cations from the phagosome, as shown using a fluorescence-based assay.
Forbes, J. R. & Gros, P. Iron, managanese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood 102, 1884–1892 (2003).
Schaible, U. E., Collins, H. L., Priem, F. & Kaufmann, S. H. E. Correction of the iron overload defect in β-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J. Exp. Med. 196, 1507–1513 (2002).
Posey, J. E. & Gherardini, F. C. Lack of a role for iron in the Lyme disease pathogen. Science 288, 1651–1653 (2000). An unprecedented demonstration of a human pathogen that has circumvented aspects of nutritional immunity by evolving to not require Fe.
Schalk, I. J. Metal trafficking via siderophores in Gram-negative bacteria: specificities and characteristics of the pyoverdine pathway. J. Inorg. Biochem. 102, 1159–1169 (2008).
Flo, T. H. et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921 (2004). The primary demonstration that NGAL-mediated binding of catecholate siderophores is crucial for the innate immune response to bacterial infection.
Abergel, R. J. et al. Anthrax pathogen evades the mammalian immune system through stealth siderophore production. Proc. Natl Acad. Sci. USA 103, 18499–18503 (2006). A molecular explanation for the observation that the Bacillus anthracis siderophore petrobactin is required for infection, whereas bacillibactin is not. The unusual 3,4-dihydroxybenzoyl chelating subunit of petrobactin prevents siderocalin from binding, establishing petrobactin as a 'stealth siderophore'.
Hantke, K., Nicholson, G., Rabsch, W. & Winkelmann, G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl Acad. Sci. USA 100, 3677–3682 (2003).
Cornelis, P. Iron uptake and metabolism in pseudomonads. Appl. Microbiol. Biotechnol. 86, 1637–1645 (2010).
Ratcliff-Griffin, M., Wilks, A. & Stojiljkovic, I. in Iron Transport in Bacteria: Molecular Genetics, Biochemistry, Microbial Pathogenesis and Ecology (eds Crosa, J. H., Mey, A. R. & Payne, S. M.) 86–94 (American Society for Microbiology Press, 2004).
Honsa, E. S. & Maresso, A. W. Mechanisms of iron import in anthrax. Biometals 24, 533–545 (2011).
Fabian, M., Solomaha, E., Olson, J. S. & Maresso, A. W. Heme transfer to the bacterial cell envelope occurs via a secreted hemophore in the Gram-positive pathogen Bacillus anthracis. J. Biol. Chem. 284, 32138–32146 (2009). A functional analysis of the only known secreted haemophore to be produced by Gram-positive pathogens.
Cescau, S. et al. Heme acquisition by hemophores. Biometals 20, 603–613 (2007).
Wilks, A. Heme oxygenase: evolution, structure, and mechanism. Antioxid. Redox Signal. 4, 603–614 (2002).
Puri, S. & O'Brian, M. R. The hmuQ and hmuD genes from Bradyrhizobium japonicum encode heme-degrading enzymes. J. Bacteriol. 188, 6476–6482 (2006).
Chim, N., Iniguez, A., Nguyen, T. Q. & Goulding, C. W. Unusual diheme conformation of the heme-degrading protein from Mycobacterium tuberculosis. J. Mol. Biol. 395, 595–608 (2010).
Haley, K. P., Janson, E. M., Heilbronner, S., Foster, T. J. & Skaar, E. P. Staphylococcus lugdunensis IsdG liberates iron from host heme. J. Bacteriol. 193, 4749–4757 (2011).
Reniere, M. L. et al. The IsdG-family of haem oxygenases degrades haem to a novel chromophore. Mol. Microbiol. 75, 1529–1538 (2010). The structural elucidation of staphylobilin, the enzymatic degradation product of the IsdG family of haem-degrading enzymes and the only product of enzymatic haem degradation that is distinct from biliverdin.
Zhang, R. et al. Crystallization and preliminary crystallographic studies of Campylobacter jejuni ChuZ, a member of a novel haem oxygenase family. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 67, 1228–1230 (2011).
Zhang, R. et al. Crystal structure of Campylobacter jejuni ChuZ: a split-barrel family heme oxygenase with a novel heme-binding mode. Biochem. Biophys. Res. Commun. 415, 82–87 (2011).
Cornelissen, C. Transferrin-iron uptake by Gram-negative bacteria. Front. Biosci. 8, D836–D847 (2003).
Aranda, J. et al. Contribution of the FeoB transporter to Streptococcus suis virulence. Int. Microbiol. 12, 137–143 (2009).
Cartron, M. L., Maddocks, S., Gillingham, P., Craven, C. J. & Andrews, S. C. Feo – transport of ferrous iron into bacteria. Biometals 19, 143–157 (2006).
Panciera, R., Marlow, D. & Stintzi, A. Major role for FeoB in Campylobacter jejuni ferrous iron acquisition, gut colonization, and intracellular survival. Infect. Immun. 74, 5433–5444 (2006).
Pandey, A. & Sonti, R. V. Role of the FeoB protein and siderophore in promoting virulence of Xanthomonas oryzae pv. oryzae on rice. J. Bacteriol. 192, 3187–3203 (2010).
Lemanceau, P., Expert, D., Gaymard, F., Bakker, P. A. H. M. & Briat, J. F. Chapter 12 Role of iron in plant–microbe interactions. Adv. Bot. Res. 51, 491–549 (2009).
Expert, D. Withholding and exchanging iron: interactions between Erwinia spp. and their plant hosts. Annu. Rev. Phytopathol. 37, 307–334 (1999).
Geiser, D. L. & Winzerling, J. J. Insect transferrins: multifunctional proteins. Biochim. Biophys. Acta 1820, 437–451 (2012).
Watson, R. J., Millichap, P., Joyce, S. A., Reynolds, S. & Clarke, D. J. The role of iron uptake in pathogenicity and symbiosis in Photorhabdus luminescens TT01. BMC Microbiol. 10, 177 (2010).
Kehl-Fie, T. E. & Skaar, E. P. Nutritional immunity beyond iron: a role for manganese and zinc. Curr. Opin. Chem. Biol. 14, 218–224 (2010).
Nies, D. H. & Grass, G. Transition metal homeostasis. In Escherichia coli and Salmonella: Cellular and Molecular Biology (eds Böck, A. et al.) chapter 5.4.4.3 EcoSal[online] (American Society for Microbiology Press, 2002).
Tseng, H. J. Srikhanta, Y., McEwan, A. G. & Jennings, M. P. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 40, 1175–1186 (2001).
Anjem, A., Varghese, S. & Imlay, J. A. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 72, 844–858 (2009).
Martin, J. E. & Imlay, J. A. The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Mol. Microbiol. 80, 319–334 (2011).
Sobota, J. M. & Imlay, J. A. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc. Natl Acad. Sci. USA 108, 5402–5407 (2011). The demonstration that crucial enzymes that utilize Fe2+ as a cofactor are primary targets of hydrogen peroxide stress, and that bacteria can protect against this stress by shifting from an Fe2+-centred to a Mn2+-centred metabolism.
Kehl-Fie, T. E. et al. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 10, 158–164 (2011). The revelation that innate immune-mediated Mn2+ chelation inactivates bacterial defences against oxidative stress at the same time that the neutrophil attacks the invading pathogen with the oxidative burst.
Hantke, K. Bacterial zinc uptake and regulators. Curr. Opin. Microbiol. 8, 196–202 (2005).
Gläser, R. et al. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nature Immunol. 6, 57–64 (2005).
Moroz, O. V. et al. Structure of the human S100A12-copper complex: implications for host-parasite defence. Acta Crystallograph. Section D Biol. Crystallogr. 59, 859–867 (2003).
Moroz, O. V. et al. Both Ca2+ and Zn2+ are essential for S100A12 protein oligomerization and function. BMC Biochem. 10, 11 (2009).
Corbin, B. D. et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962–965 (2008). The first demonstration that calprotectin protects against infection through nutrient metal chelation, establishing calprotectin as the only known Mn2+-chelating protein of the innate immune system.
McCormick, A. et al. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect. 12, 928–936 (2010).
Urban, C. F. et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5, e1000639 (2009). The seminal finding that calprotectin is an abundant component of neutrophil extracellular traps (NETs) and protects against fungal infection.
Liu, J. Z. et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11, 227–239 (2012). The first description of a bacterial pathogen exploiting calprotectin to provide a growth advantage over competing commensal bacteria. Specifically, S . Typhimurium uses high-affinity Zn2+ acquisition systems to overcome calprotectin-mediated Zn2+ chelation and thrive in the inflamed gut.
Bianchi, M., Niemiec, M. J., Siler, U., Urban, C. F. & Reichenbach, J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J. Allergy Clin. Immunol. 127, 1243–1252.e7 (2011).
Hsu, K. et al. Anti-infective protective properties of S100 calgranulins. AntiInflamm. Antiallergy Agents Med. Chem. 8, 290–305 (2009).
Kehres, D. G., Janakiraman, A., Slauch, J. M. & Maguire, M. E. SitABCD is the alkaline Mn2+ transporter of Salmonella enterica serovar typhimurium. J. Bacteriol. 184, 3159–3166 (2002).
Ammendola, S. et al. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect. Immun. 75, 5867–5876 (2007).
Campoy, S. et al. Role of the high-affinity zinc uptake znuABC system in Salmonella enterica serovar typhimurium virulence. Infect. Immun. 70, 4721–4725 (2002).
Davis, L. M., Kakuda, T. & DiRita, V. J. A Campylobacter jejuni znuA orthologue is essential for growth in low-zinc environments and chick colonization. J. Bacteriol. 191, 1631–1640 (2009).
Bearden, S. W. & Perry, R. D. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol. Microbiol. 32, 403–414 (1999).
Champion, O. L. et al. Yersinia pseudotuberculosis mntH functions in intracellular manganese accumulation, which is essential for virulence and survival in cells expressing functional Nramp1. Microbiology 157, 1115–1122 (2011).
Perry, R. D. et al. Manganese transporters Yfe and MntH are Fur regulated and important for the virulence of Yersinia pestis. Microbiology 158, 804–815 (2012).
Anderson, E. S. et al. The manganese transporter MntH is a critical virulence determinant for Brucella abortus 2308 in experimentally infected mice. Infect. Immun. 77, 3466–3474 (2009).
Rosadini, C. V., Gawronski, J. D., Raimunda, D., Argüello, J. M. & Akerley, B. J. A novel zinc binding system, ZevAB, is critical for survival of nontypeable Haemophilus influenzae in a murine lung infection model. Infect. Immun. 79, 3366–3376 (2011).
Corbett, D. et al. Two zinc uptake systems contribute to the full virulence of Listeria monocytogenes during growth in vitro and in vivo. Infect. Immun. 80, 14–21 (2012).
Bayle, L. et al. Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Mol. Microbiol. 82, 904–916 (2011).
Hohle, T. H., Franck, W. L., Stacey, G. & O'Brian, M. R. Bacterial outer membrane channel for divalent metal ion acquisition. Proc. Natl Acad. Sci. USA 108, 15390–15395 (2011).
Stork, M. et al. An outer membrane receptor of Neisseria meningitidis involved in zinc acquisition with vaccine potential. PLoS Pathog. 6, e1000969 (2010).
Kumar, P., Sannigrahi, S. & Tzeng, Y.-L. The Neisseria meningitidis ZnuD zinc receptor contributes to interactions with epithelial cells and supports heme utilization when expressed in Escherichia coli. Infect. Immun. 80, 657–667 (2012).
Li, J. M., Russell, C. S. & Cosloy, S. D. The structure of the Escherichia coli hemB gene. Gene 75, 177–184 (1989).
Kallifidas, D. et al. The zinc-responsive regulator Zur controls expression of the coelibactin gene cluster in Streptomyces coelicolor. J. Bacteriol. 192, 608–611 (2010).
Brandel, J. et al. Pyochelin, a siderophore of Pseudomonas aeruginosa: physicochemical characterization of the iron(III), copper(II) and zinc(II) complexes. Dalton Trans. 41, 2820–2834 (2012).
Klein, J. S. & Lewinson, O. Bacterial ATP-driven transporters of transition metals: physiological roles, mechanisms of action, and roles in bacterial virulence. Metallomics 3, 1098–1108 (2011).
Andresen, E. et al. S100A7/psoriasin expression in the human lung: unchanged in patients with COPD, but upregulated upon positive S. aureus detection. BMC Pulm. Med. 11, 10 (2011).
Nielubowicz, G. R., Smith, S. N. & Mobley, H. L. T. Zinc uptake contributes to motility and provides a competitive advantage to Proteus mirabilis during experimental urinary tract infection. Infect. Immun. 78, 2823–2833 (2010).
Dashper, S. G. et al. A novel Porphyromonas gingivalis FeoB plays a role in manganese accumulation. J. Biol. Chem. 280, 28095–28102 (2005).
Botella, H., Stadthagen, G., Lugo-Villarino, G., de Chastellier, C. & Neyrolles, O. Metallobiology of host–pathogen interactions: an intoxicating new insight. Trends Microbiol. 20, 106–112 (2012).
Botella, H. et al. Mycobacterial P1-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10, 248–259 (2011). The surprising findings that Zn2+ is directed at M. tuberculosis within the phagosome, and that M. tuberculosis neutralizes the toxic effects of Zn2+ accumulation through efflux.
Hou, Z. J., Narindrasorasak, S., Bhushan, B., Sarkar, B. & Mitra, B. Functional analysis of chimeric proteins of the Wilson Cu(I)-ATPase (ATP7B) and ZntA, a Pb(II)/Zn(II)/Cd(II)-ATPase from Escherichia coli. J. Biol. Chem. 276, 40858–40863 (2001).
Veyrier, F. J., Boneca, I. G., Cellier, M. F. & Taha, M.-K. A novel metal transporter mediating manganese export (MntX) regulates the Mn to Fe intracellular ratio and Neisseria meningitidis virulence. PLoS Pathog. 7, e1002261 (2011).
Rosch, J. W., Gao, G., Ridout, G., Wang, Y.-D. & Tuomanen, E. I. Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol. Microbiol. 72, 12–25 (2009).
Jacobsen, F. E., Kazmierczak, K. M., Lisher, J. P., Winkler, M. E. & Giedroc, D. P. Interplay between manganese and zinc homeostasis in the human pathogen Streptococcus pneumoniae. Metallomics 3, 38–41 (2011).
McDevitt, C. A. et al. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog. 7, e1002357 (2011).
Dintilhac, A., Alloing, G., Granadel, C. & Claverys, J.-P. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25, 727–739 (1997).
Lawrence, M. C. et al. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 6, 1553–1561 (1998).
Ogunniyi, A. D. et al. Central role of manganese in regulation of stress responses, physiology, and metabolism in Streptococcus pneumoniae. J. Bacteriol. 192, 4489–4497 (2010).
Samanovic, M. I., Ding, C., Thiele, D. J. & Darwin, K. H. Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe 11, 106–115 (2012).
Wolschendorf, F. et al. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 108, 1621–1626 (2011). The report that Cu transport proteins are crucial for mycobacterial Cu resistance and infection in animal models.
White, C., Lee, J., Kambe, T., Fritsche, K. & Petris, M. J. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem. 284, 33949–33956 (2009).
Kim, B.-E., Nevitt, T. & Thiele, D. J. Mechanisms for copper acquisition, distribution and regulation. Nature Chem. Biol. 4, 176–185 (2008).
Kim, H. W. et al. Human macrophage ATP7A is localized in the trans-Golgi apparatus, controls intracellular copper levels, and mediates macrophage responses to dermal wounds. Inflammation 35, 167–175 (2011).
Macomber, L., Rensing, C. & Imlay, J. A. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J. Bacteriol. 189, 1616–1626 (2007).
Djoko, K. Y. et al. Phenotypic characterization of a copA mutant of Neisseria gonorrhoeae identifies a link between copper and nitrosative stress. Infect. Immun. 80, 1065–1071 (2012).
Edwards, J. L. Neisseria gonorrhoeae survival during primary human cervical epithelial cell infection requires nitric oxide and is augmented by progesterone. Infect. Immun. 78, 1202–1213 (2010).
Xu, F. F. & Imlay, J. A. Silver(I), mercury(II), cadmium(II), and zinc(II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl. Environ. Microbiol. 78, 3614–3621 (2012).
Achard, M. E. S. et al. The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect. Immun. 78, 2312–2319 (2010).
Macomber, L. & Imlay, J. A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl Acad. Sci. USA 106, 8344–8349 (2009).
Knapp, C. W., Fowle, D. A., Kulczycki, E., Roberts, J. A. & Graham, D. W. Methane monooxygenase gene expression mediated by methanobactin in the presence of mineral copper sources. Proc. Natl Acad. Sci. USA 104, 12040–12045 (2007).
Hakemian, A. S. & Rosenzweig, A. C. The biochemistry of methane oxidation. Annu. Rev. Biochem. 76, 223–241 (2007).
Kenney, G. E. & Rosenzweig, A. C. Chemistry and biology of the copper chelator methanobactin. ACS Chem. Biol. 7, 260–268 (2011).
Kim, H. J. Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science 305, 1612–1615 (2004).
Balasubramanian, R. & Rosenzweig, A. C. Copper methanobactin: a molecule whose time has come. Curr. Opin. Chem. Biol. 12, 245–249 (2008).
Hakemian, A. S. et al. The copper chelator methanobactin from Methylosinus trichosporium OB3b binds copper(I). J. Am. Chem. Soc. 127, 17142–17143 (2005).
El Ghazouani, A. et al. Copper-binding properties and structures of methanobactins from Methylosinus trichosporium OB3b. Inorg. Chem. 50, 1378–1391 (2011).
Balasubramanian, R., Kenney, G. E. & Rosenzweig, A. C. Dual pathways for copper uptake by methanotrophic bacteria. J. Biol. Chem. 286, 37313–37319 (2011).
Shafeeq, S. et al. The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol. Microbiol. 81, 1255–1270 (2011).
Sharan, R., Chhibber, S. & Reed, R. H. A murine model to study the antibacterial effect of copper on infectivity of Salmonella enterica serovar Typhimurium. Int. J. Environ. Res. Public Health 8, 21–36 (2011).
Ward, S. K., Abomoelak, B., Hoye, E. A., Steinberg, H. & Talaat, A. M. CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol. Microbiol. 77, 1096–1110 (2010).
Liu, T. et al. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nature Chem. Biol. 3, 60–68 (2006). A seminal paper describing the identification of a large, previously uncharacterized family of transcriptional regulators, highlighted by the Cu+-specific repressor CsoR.
Festa, R. A. et al. A novel copper-responsive regulon in Mycobacterium tuberculosis. Mol. Microbiol. 79, 133–148 (2010).
Kim, J. S. et al. The sctR of Salmonella enterica serova Typhimurium encoding a homologue of MerR protein is involved in the copper-responsive regulation of cuiD. FEMS Microbiol. Lett. 210, 99–103 (2002).
Osman, D. et al. Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J. Biol. Chem. 285, 25259–25268 (2010).
Outten, F. W., Huffman, D. L., Hale, J. A. & O'Halloran, T. V. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 276, 30670–30677 (2001).
Lu, Z. H., Dameron, C. T. & Solioz, M. The Enterococcus hirae paradigm of copper homeostasis: copper chaperone turnover, interactions, and transactions. Biometals 16, 137–143 (2003).
Hsu, Y.-H. et al. Association of NRAMP 1 gene polymorphism with susceptibility to tuberculosis in Taiwanese aboriginals. J. Formos. Med. Assoc. 105, 363–369 (2006).
Huang, J. H. et al. Analyses of the NRAMP1 and IFN-γR1 genes in women with Mycobacterium avium-intracellulare pulmonary disease. Am. J. Respir. Crit. Care Med. 157, 377–381 (1998).
Tanaka, G. et al. Pulmonary Mycobacterium avium complex infection: association with NRAMP1 polymorphisms. Eur. Respir. J. 30, 90–96 (2007).
Isidor, B. et al. Hyperzincemia and hypercalprotectinemia: unsuccessful treatment with tacrolimus. Acta Paediatr. 98, 410–412 (2009).
Saito, Y. et al. Hyperzincemia with systemic inflammation: a heritable disorder of calprotectin metabolism with rheumatic manifestations? J. Pediatr. 140, 267–269 (2002).
Lee, A. C. W. & Li, C. H. Age as a factor in severe bacterial infection in transfusion-dependent patients with thalassemia major. Clin. Infect. Dis. 38, 1194–1195; author reply 1195 (2004).
Wang, S.-C. et al. Severe bacterial infection in transfusion-dependent patients with thalassemia major. Clin. Infect. Dis. 37, 984–988 (2003).
Gerhard, G. S. et al. Vibrio vulnificus septicemia in a patient with the hemochromatosis HFE C282Y mutation. Arch. Pathol. Lab. Med. 125, 1107–1109 (2001).
Höpfner, M. et al. Yersinia enterocolitica infection with multiple liver abscesses uncovering a primary hemochromatosis. Scand. J. Gastroenterol. 36, 220–224 (2001).
Weinberg, E. D. Survival advantage of the hemochromatosis C282Y mutation. Perspect. Biol. Med. 51, 98–102 (2008).
Pishchany, G. et al. Specificity for human hemoglobin enhances Staphylococcus aureus infection. Cell Host Microbe 8, 544–550 (2010). Evidence that polymorphisms within haemoglobin affect susceptibility to S. aureus infections.
Torres, V. J., Pishchany, G., Humayun, M., Schneewind, O. & Skaar, E. P. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J. Bacteriol. 188, 8421–8429 (2006).
Krishna Kumar, K. et al. Structural basis for hemoglobin capture by Staphylococcus aureus cell-surface protein, IsdH. J. Biol. Chem. 286, 38439–38447 (2011).
Zarantonelli, M.-L. et al. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect. Immun. 75, 5609–5614 (2007).
Schryvers, A. B. & Morris, L. J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol. Microbiol. 2, 281–288 (1988).
Noinaj, N. et al. Structural basis for iron piracy by pathogenic Neisseria. Nature 483, 53–58 (2012). The crystal structure of the Neisseria spp. TbpA in complex with transferrin.
Calmettes, C., Alcantara, J., Yu, R.-H., Schryvers, A. B. & Moraes, T. F. The structural basis of transferrin sequestration by transferrin-binding protein B. Nature Struct. Mol. Biol. 19, 358–360 (2012).
Borkow, G. & Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 12, 2163–2175 (2005).
Mikolay, A. et al. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl. Microbiol. Biotechnol. 87, 1875–1879 (2010).
Casey, A. L. et al. Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 74, 72–77 (2010).
Zhou, T., Ma, Y., Kong, X. & Hider, R. C. Design of iron chelators with therapeutic application. Dalton Trans. 41, 6371–6389 (2012).
Summer, K. H. et al. The biogenic methanobactin is an effective chelator for copper in a rat model for Wilson disease. J. Trace Elem. Med. Biol. 25, 36–41 (2011).
Thiennimitr, P. et al. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl Acad. Sci. USA 108, 17480–17485 (2011).
Winter, S. E. et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467, 426–429 (2010).
Hoffman, L. R. et al. Nutrient availability as a mechanism for selection of antibiotic tolerant Pseudomonas aeruginosa within the CF airway. PLoS Pathog. 6, e1000712 (2010).
Ponton, F., Wilson, K., Cotter, S. C., Raubenheimer, D. & Simpson, S. J. Nutritional immunology: a multi-dimensional approach. PLoS Pathog. 7, e1002223 (2011).
Rohmer, L., Hocquet, D. & Miller, S. I. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol. 19, v341–348 (2011).
Eisenreich, W., Dandekar, T., Heesemann, J. & Goebel, W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nature Rev. Microbiol. 8, 401–412 (2010).
Price, C. T. D., Al-Quadan, T., Santic, M., Rosenshine, I. & Abu Kwaik, Y. Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science 334, 1553–1557 (2011).
Segond, D. et al. NRAMP genes function in Arabidopsis thaliana resistance to Erwinia chrysanthemi infection. Plant J. 58, 195–207 (2009).
Dellagi, A. et al. Microbial siderophores exert a subtle role in Arabidopsis during infection by manipulating the immune response and the iron status. Plant Physiol. 150, 1687–1696 (2009).
Rokhbakhsh-Zamin, F. et al. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J. Microbiol. Biotechnol. 21, 556–566 (2011).
Lemanceau, P., Bauer, P., Kraemer, S. & Briat, J.-F. Iron dynamics in the rhizosphere as a case study for analyzing interactions between soils, plants and microbes. Plant Soil 321, 513–535 (2009).
Cheung, J., Beasley, F. C., Liu, S., Lajoie, G. A. & Heinrichs, D. E. Molecular characterization of staphyloferrin B biosynthesis in Staphylococcus aureus. Mol. Microbiol. 74, 594–608 (2009).
Beasley, F. C. et al. Characterization of staphyloferrin A biosynthetic and transport mutants in Staphylococcus aureus. Mol. Microbiol. 72, 947–963 (2009).
Acknowledgements
The authors thank members of the Skaar laboratory for critical reading of the manuscript. Work in the Skaar laboratory is supported by grants AI091771, AI069233 and AI073843 from the US National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. E.P.S. is a Burroughs Wellcome Investigator in the Pathogenesis of Infectious Diseases. M.I.H. was supported by a Howard Hughes International Student Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Transition metals
-
Elements that are in groups 3–12 of the periodic table, have an incomplete inner (penultimate) electron shell and can therefore exhibit multiple valences.
- Protoporphyrin
-
A tetrapyrole ring containing two vinyl, four methyl and two propionic acid side chains encircling a metal ion. In the case of haem, the tetrapyrrole ring encircles a singular Fe2+ atom.
- Haptoglobin
-
A serum protein that binds free haemoglobin and inhibits its oxidative activity.
- Haemopexin
-
A haem-scavenging protein that is found in serum and binds haem with high affinity.
- Natural resistance-associated macrophage protein 1
-
A divalent cation transporter that is expressed on the phagosomal membrane.
- Biliverdin
-
A green pigment that is a product of enzymatic haem catabolism.
- Staphylobilin
-
A product of haem catabolism; this molecule is produced by the IsdG family of haem oxygenases.
- SH3 domain protein
-
Proteins containing the SRC homology 3 (SH3) domain, which consists of five or six β-strands arranged as two tightly packed β-sheets. This domain typically mediates protein–protein interactions by binding to proline-rich regions on the binding partner.
- Fe–S clusters
-
Complexes of Fe and bridging sulphides. Fe–S clusters are often found in metalloproteins and have structural or functional roles in proteins, most notably in electron transfer reactions and redox sensing.
- Rhizosphere
-
The zone immediately surrounding the plant root; in this zone, biological and chemical interactions occur among the plant, the soil itself and soil microorganisms.
- Superoxide dismutase
-
An enzyme that catalyses the formation of hydrogen peroxide from superoxide.
- Pyochelin
-
A siderophore that is produced by Pseudomonas spp. and binds Fe3+ and some other metal ions with high affinity.
- P type ATPases
-
A class of autocatalytic ATP-hydrolysing transporters that is found in bacteria, archaea and eukaryotes. Most members of this class transport cations.
- RND family transporters
-
Efflux transporters that span the inner and outer membranes of Gram-negative bacteria. These transporters harness the proton gradient at the inner membrane to drive substrate efflux from the cytosol to the extracellular environment.
- Fenton chemistry
-
The Fe2+-catalysed production of hydroxyl radicals from hydrogen peroxide: Fe2++H2O2→Fe3++OH•+OH−.
- Haemochromatosis
-
A condition of Fe overload that can result from a primary defect in Fe absorption or storage, or that can occur secondarily to medical procedures such as blood transfusions.
Rights and permissions
About this article
Cite this article
Hood, M., Skaar, E. Nutritional immunity: transition metals at the pathogen–host interface. Nat Rev Microbiol 10, 525–537 (2012). https://doi.org/10.1038/nrmicro2836
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2836
This article is cited by
-
Spectroscopic and computational investigations of Cobalt(II) binding to the innate immune protein human calprotectin
JBIC Journal of Biological Inorganic Chemistry (2024)
-
Nutritional Immunity and Fungal Pathogens: A New Role for Manganese
Current Clinical Microbiology Reports (2024)
-
Pulcherriminic acid modulates iron availability and protects against oxidative stress during microbial interactions
Nature Communications (2023)
-
Humoral regulation of iron metabolism by extracellular vesicles drives antibacterial response
Nature Metabolism (2023)
-
Clostridioides difficile ferrosome organelles combat nutritional immunity
Nature (2023)