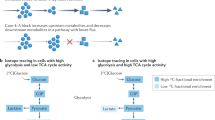
Key Points
-
Stem cells perpetuate themselves through self-renewal and they replenish mature cells to maintain tissue homeostasis throughout the lifespan of an organism.
-
Stem cell function is precisely regulated by various intrinsic mechanisms, in coordination with extrinsic stimuli; recent studies have revealed critical roles for stem cell metabolism in maintaining stem cell self-renewal.
-
Stem cells reside within specialized niches (for example, a hypoxic niche), where specific local conditions have a role in maintaining a quiescent state that is essential for preserving the self-renewal capacity of stem cells.
-
Many types of stem cells heavily rely on anaerobic glycolysis, rather than mitochondrial oxidative phosphorylation, to produce the low levels of intracellular reactive oxygen species, which inhibit stem cell ageing.
-
The balance between stem cell quiescence and proliferation is regulated by the nutrient-sensitive PI3K–AKT–mTOR and AMPK pathways, and Gln metabolism.
-
Recent studies have uncovered a crucial role for fatty acid metabolism in the self-renewal of haematopoietic stem cells (HSCs) through the control it exerts over stem cell fate decisions.
Abstract
A distinctive feature of stem cells is their capacity to self-renew to maintain pluripotency. Studies of genetically-engineered mouse models and recent advances in metabolomic analysis, particularly in haematopoietic stem cells, have deepened our understanding of the contribution made by metabolic cues to the regulation of stem cell self-renewal. Many types of stem cells heavily rely on anaerobic glycolysis, and stem cell function is also regulated by bioenergetic signalling, the AKT–mTOR pathway, Gln metabolism and fatty acid metabolism. As maintenance of a stem cell pool requires a finely-tuned balance between self-renewal and differentiation, investigations into the molecular mechanisms and metabolic pathways underlying these decisions hold great therapeutic promise.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Weissman, I. L., Anderson, D. J. & Gage, F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 17, 387–403 (2001).
Seita, J. & Weissman, I. L. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 640–653 (2010).
Zon, L. I. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature 453, 306–313 (2008).
He, S., Nakada, D. & Morrison, S. J. Mechanisms of stem cell self-renewal. Annu. Rev. Cell Dev. Biol. 25, 377–406 (2009).
van der Lugt, N. M. et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 8, 757–769 (1994).
Morrison, S. J. & Kimble, J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441, 1068–1074 (2006).
Lansdorp, P. M. Intrinsic control of stem cell fate. Stem Cells 15 (Suppl. 1), 223–225; discussion 225–227 (1997).
Suda, T., Suda, J. & Ogawa, M. Single-cell origin of mouse hemopoietic colonies expressing multiple lineages in variable combinations. Proc. Natl Acad. Sci. USA 80, 6689–6693 (1983).
Suda, T., Suda, J. & Ogawa, M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc. Natl Acad. Sci. USA 81, 2520–2524 (1984).
Metcalf, D. Lineage commitment in the progeny of murine hematopoietic preprogenitor cells: influence of thrombopoietin and interleukin 5. Proc. Natl Acad. Sci. USA 95, 6408–6412 (1998).
Tothova, Z. & Gilliland, D. G. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell 1, 140–152 (2007).
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997–1002 (2004).
Ito, K. et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Med. 12, 446–451 (2006).
Miyamoto, K. et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1, 101–112 (2007).
Figueroa, M. E. et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553–567 (2010).
Gan, B. et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature 468, 701–704 (2010).
Gurumurthy, S. et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature 468, 659–663 (2010).
Nakada, D., Saunders, T. L. & Morrison, S. J. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 468, 653–658 (2010). Together with references 16 and 17, this study demonstrates that the protein LKB1, which lies at the crossroad of energy metabolism and cell growth, seems to regulate HSC dynamics.
Tefferi, A. et al. IDH1 and IDH2 mutation studies in 1473 patients with chronic-, fibrotic- or blast-phase essential thrombocythemia, polycythemia vera or myelofibrosis. Leukemia 24, 1302–1309 (2010).
Ward, P. S. et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17, 225–234 (2010).
Lu, C. et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483, 474–478 (2012).
Ito, K. et al. A PML–PPAR-δe pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nature Med. 18, 1350–1358 (2012). This study shows that PML acts as a critical rheostat responsible for fine-tuning tissue homeostasis.
Suda, T., Takubo, K. & Semenza, G. L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 9, 298–310 (2011). A comprehensive review on oxygen homeostasis, the hypoxic niche and energy metabolism for maintenance of HSC function and long-term self-renewal.
Orkin, S. H. & Zon, L. I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644 (2008).
Shyh-Chang, N., Daley, G. Q. & Cantley, L. C. Stem cell metabolism in tissue development and aging. Development 140, 2535–2547 (2013). An important review of recent studies of the balance among glycolysis, mitochondrial OXPHOS and oxidative stress in various forms of stem cells.
Xu, X. et al. Mitochondrial regulation in pluripotent stem cells. Cell. Metab. 18, 325–332 (2013).
Mohyeldin, A., Garzon-Muvdi, T. & Quinones-Hinojosa, A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7, 150–161 (2010).
Simon, M. C. & Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nature Rev. Mol. Cell Biol. 9, 285–296 (2008).
Nombela-Arrieta, C. et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nature Cell Biol. 15, 533–543 (2013).
Simsek, T. et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7, 380–390 (2010).
Suda, T. Hematopoiesis and bone remodeling. Blood 117, 5556–5557 (2011).
Takubo, K. et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 12, 49–61 (2013). This study demonstrates that glycolytic metabolic status governed by PDK acts as a cell cycle checkpoint that modulates HSC quiescence and function.
Warr, M. R. & Passegue, E. Metabolic makeover for HSCs. Cell Stem Cell 12, 1–3 (2013).
Yu, W. M. et al. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell 12, 62–74 (2013).
Semenza, G. L. Oxygen homeostasis. Wiley Interdiscip Rev. Syst. Biol. Med. 2, 336–361 (2010).
Wang, G. L. & Semenza, G. L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 270, 1230–1237 (1995).
Wang, G. L., Jiang, B. H., Rue, E. A. & Semenza, G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA 92, 5510–5514 (1995).
Cavadas, M. A., Nguyen, L. K. & Cheong, A. Hypoxia-inducible factor (HIF) network: insights from mathematical models. Cell Commun. Signal 11, 42 (2013).
Nguyen, L. K. et al. A dynamic model of the hypoxia-inducible factor 1α (HIF-1α) network. J. Cell Sci. 126, 1454–1463 (2013).
Bruick, R. K. & McKnight, S. L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 (2001).
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001).
Ivan, M. et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc. Natl Acad. Sci. USA 99, 13459–13464 (2002).
Barger, J. F. & Plas, D. R. Balancing biosynthesis and bioenergetics: metabolic programs in oncogenesis. Endocr. Relat. Cancer 17, R287–304 (2010).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer 3, 721–732 (2003).
Simon, M. C., Liu, L., Barnhart, B. C. & Young, R. M. Hypoxia-induced signaling in the cardiovascular system. Annu. Rev. Physiol. 70, 51–71 (2008).
Rouault-Pierre, K. et al. HIF-2α protects human hematopoietic stem/progenitors and acute myeloid leukemic cells from apoptosis induced by endoplasmic reticulum stress. Cell Stem Cell 13, 549–563 (2013).
Gu, Y. Z., Moran, S. M., Hogenesch, J. B., Wartman, L. & Bradfield, C. A. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3α. Gene Expr 7, 205–213 (1998).
Makino, Y., Kanopka, A., Wilson, W. J., Tanaka, H. & Poellinger, L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3α locus. J. Biol. Chem. 277, 32405–32408 (2002).
Kranc, K. R. et al. Cited2 is an essential regulator of adult hematopoietic stem cells. Cell Stem Cell 5, 659–665 (2009).
Du, J. et al. HIF-1α deletion partially rescues defects of hematopoietic stem cell quiescence caused by Cited2 deficiency. Blood 119, 2789–2798 (2012).
Du, J. et al. Cited2 is required for the maintenance of glycolytic metabolism in adult hematopoietic stem cells. Stem Cells Dev. 23, 83–94 (2014).
Miharada, K. et al. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell Stem Cell 9, 330–344 (2011).
Miharada, K. et al. Hematopoietic stem cells are regulated by Cripto, as an intermediary of HIF-1αin the hypoxic bone marrow niche. Ann. NY Acad. Sci. 1266, 55–62 (2012).
Rossi, D. J., Jamieson, C. H. & Weissman, I. L. Stems cells and the pathways to aging and cancer. Cell 132, 681–696 (2008).
Chen, C. T., Shih, Y. R., Kuo, T. K., Lee, O. K. & Wei, Y. H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 26, 960–968 (2008).
Pattappa, G., Heywood, H. K., de Bruijn, J. D. & Lee, D. A. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J. Cell. Physiol. 226, 2562–2570 (2011).
Pattappa, G. et al. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng. Part C Methods 19, 68–79 (2013).
Renault, V. M. et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 5, 527–539 (2009).
Van Blerkom, J. Mitochondria in early mammalian development. Semin. Cell Dev. Biol. 20, 354–364 (2009).
Kondoh, H. et al. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid. Redox Signal 9, 293–299 (2007).
Shyh-Chang, N. et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339, 222–226 (2013).
Folmes, C. D. et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell. Metab. 14, 264–271 (2011).
Varum, S. et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE 6, e20914 (2011).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Peng, S. et al. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells 29, 496–504 (2011).
Balzer, E. & Moss, E. G. Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol. 4, 16–25 (2007).
Viswanathan, S. R., Daley, G. Q. & Gregory, R. I. Selective blockade of microRNA processing by Lin28. Science 320, 97–100 (2008).
Shyh-Chang, N. & Daley, G. Q. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell 12, 395–406 (2013).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Yuan, J., Nguyen, C. K., Liu, X., Kanellopoulou, C. & Muljo, S. A. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 335, 1195–1200 (2012).
Zheng, K., Wu, X., Kaestner, K. H. & Wang, P. J. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev. Biol. 9, 38 (2009).
Shyh-Chang, N. et al. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell 155, 778–792 (2013).
Zhu, H. et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 147, 81–94 (2011).
Zhang, J. et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 30, 4860–4873 (2011).
Shyh-Chang, N., Zheng, Y., Locasale, J. W. & Cantley, L. C. Human pluripotent stem cells decouple respiration from energy production. EMBO J. 30, 4851–4852 (2011).
Forman, N. G. & Wilson, D. F. Energetics and stoichiometry of oxidative phosphorylation from NADH to cytochrome c in isolated rat liver mitochondria. J. Biol. Chem. 257, 12908–12915 (1982).
Wang, J. et al. Dependence of mouse embryonic stem cells on threonine catabolism. Science 325, 435–439 (2009).
Manganelli, G. et al. Modulation of the pentose phosphate pathway induces endodermal differentiation in embryonic stem cells. PLoS ONE 7, e29321 (2012).
Kathagen, A. et al. Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol. 126, 763–80 (2013).
Zhao, F. et al. Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1α-induced metabolic reprograming. Oncogene 29, 2962–2972 (2010).
Cho, Y. M. et al. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 348, 1472–1478 (2006).
Chung, S., Arrell, D. K., Faustino, R. S., Terzic, A. & Dzeja, P. P. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J. Mol. Cell Cardiol 48, 725–734 (2010).
Prigione, A., Fauler, B., Lurz, R., Lehrach, H. & Adjaye, J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 28, 721–733 (2010).
St John, J. C. et al. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Clon. Stem Cells 7, 141–153 (2005).
Norddahl, G. L. et al. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell 8, 499–510 (2011).
Inoue, S. et al. Mitochondrial respiration defects modulate differentiation but not proliferation of hematopoietic stem and progenitor cells. FEBS Lett. 584, 3402–3409 (2010).
Maryanovich, M. et al. The ATM-BID pathway regulates quiescence and survival of haematopoietic stem cells. Nature Cell Biol. 14, 535–541 (2012).
Maryanovich, M. & Gross, A. A. ROS rheostat for cell fate regulation. Trends Cell Biol. 23, 129–134 (2013).
Weiss, C. N. & Ito, K. DNA damage response, redox status and hematopoiesis. Blood Cells Mol. Dis. 52, 12–18 (2013).
Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007). Together with references 11 and 14, this study demonstrates an important role of FOXO proteins in the maintenance and integrity of stem cells.
Paik, J. H. et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128, 309–323 (2007).
Yamazaki, S. et al. Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J. 25, 3515–3523 (2006).
Paik, J. H. et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 5, 540–553 (2009).
Hagenbuchner, J. & Ausserlechner, M. J. Mitochondria and FOXO3: breath or die. Front. Physiol. 4, 147 (2013).
Mortensen, M. et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J. Exp. Med. 208, 455–467 (2011).
Warr, M. R. et al. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature 494, 323–327 (2013).
Barzilai, A., Rotman, G. & Shiloh, Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair 1, 3–25 (2002).
Allen, D. M. et al. Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev. 15, 554–566 (2001).
Jang, Y. Y. & Sharkis, S. J. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110, 3056–3063 (2007).
Nitta, E. et al. Telomerase reverse transcriptase protects ATM-deficient hematopoietic stem cells from ROS-induced apoptosis through a telomere-independent mechanism. Blood 117, 4169–4180 (2011).
Kaplon, J. et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 498, 109–112 (2013).
Harris, J. M. et al. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood 121, 2483–2493 (2013).
Morimoto, H. et al. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell 12, 774–786 (2013).
Lewandowski, D. et al. In vivo cellular imaging pinpoints the role of reactive oxygen species in the early steps of adult hematopoietic reconstitution. Blood 115, 443–452 (2010).
Yuan, T. L. & Cantley, L. C. PI3K pathway alterations in cancer: variations on a theme. Oncogene 27, 5497–5510 (2008).
Kharas, M. G. & Gritsman, K. Akt: a double-edged sword for hematopoietic stem cells. Cell Cycle 9, 1223–1224 (2010).
Kharas, M. G. et al. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood 115, 1406–1415 (2010).
Yilmaz, O. H. et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 (2006).
Zhang, J. et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 441, 518–522 (2006).
Lee, J. Y. et al. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell 7, 593–605 (2010). In this study, mTOR activation depletes HSCs through a tumour suppressor response.
Chen, C. et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 205, 2397–2408 (2008).
Gan, B. et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc. Natl Acad. Sci. USA 105, 19384–19389 (2008).
Groszer, M. et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294, 2186–2189 (2001).
Zhou, J. et al. Tsc1 mutant neural stem/progenitor cells exhibit migration deficits and give rise to subependymal lesions in the lateral ventricle. Genes Dev. 25, 1595–1600 (2011).
Yilmaz, O. H. et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486, 490–495 (2012).
Shackelford, D. B. & Shaw, R. J. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nature Rev. Cancer 9, 563–575 (2009).
Hsu, P. P. & Sabatini, D. M. Cancer cell metabolism: Warburg and beyond. Cell 134, 703–707 (2008).
Wise, D. R. & Thompson, C. B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 35, 427–433 (2010).
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013).
Dang, C. V. et al. The c-Myc target gene network. Semin. Cancer Biol. 16, 253–264 (2006).
Carracedo, A. et al. A metabolic prosurvival role for PML in breast cancer. J. Clin. Invest. 122, 3088–3100 (2012).
Carracedo, A., Cantley, L. C. & Pandolfi, P. P. Cancer metabolism: fatty acid oxidation in the limelight. Nature Rev. Cancer 13, 227–232 (2013).
Schafer, Z. T. et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461, 109–113 (2009).
Dunning, K. R. et al. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol. Reprod. 83, 909–918 (2010).
Zaugg, K. et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 25, 1041–1051 (2011).
Ito, K. et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature 453, 1072–1078 (2008).
Kiel, M. J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Arai, F. et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161 (2004).
Ito, K. & Ito, K. Newly Identified Roles of PML in Stem Cell Biology. Front. Oncol. 3, 50 (2013).
Riserus, U. et al. Activation of peroxisome proliferator-activated receptor (PPAR) δ promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes 57, 332–339 (2008).
Berger, J. P., Akiyama, T. E. & Meinke, P. T. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol. Sci. 26, 244–251 (2005).
Wagner, K. D. & Wagner, N. Peroxisome proliferator-activated receptor beta/delta (PPARβ/δ) acts as regulator of metabolism linked to multiple cellular functions. Pharmacol. Ther. 125, 423–435 (2010).
Samudio, I. et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J. Clin. Invest. 120, 142–156 (2010).
Hosokawa, K. et al. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem. Biophys. Res. Commun. 363, 578–583 (2007).
Pike, L. S., Smift, A. L., Croteau, N. J., Ferrick, D. A. & Wu, M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim. Biophys. Acta 1807, 726–734 (2011).
Jeon, S. M., Chandel, N. S. & Hay, N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485, 661–665 (2012).
Caro, P. et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell 22, 547–560 (2012).
Knobloch, M. et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature 493, 226–230 (2013).
Galdieri, L. & Vancura, A. Acetyl-CoA carboxylase regulates global histone acetylation. J. Biol. Chem. 287, 23865–23876 (2012).
Lerit, D. A., Smyth, J. T. & Rusan, N. M. Organelle asymmetry for proper fitness, function, and fate. Chromosome Res. 21, 271–286 (2013).
Filosa, S. et al. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem. J. 370, 935–943 (2003).
Christofk, H. R. et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 (2008).
Israelsen, W. J. et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell 155, 397–409 (2013).
Anastasiou, D. et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283 (2011).
Shih, A. H., Abdel-Wahab, O., Patel, J. P. & Levine, R. L. The role of mutations in epigenetic regulators in myeloid malignancies. Nature Rev. Cancer 12, 599–612 (2012).
Jones, P. A. & Baylin, S. B. The fundamental role of epigenetic events in cancer. Nature Rev. Genet. 3, 415–428 (2002).
Viswanathan, S. R. & Daley, G. Q. Lin28: A microRNA regulator with a macro role. Cell 140, 445–449 (2010).
Song, S. J. et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via tet-family-dependent chromatin remodeling. Cell 154, 311–324 (2013).
Song, S. J. et al. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell 13, 87–101 (2013).
Zhu, H. et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nature Genet. 42, 626–630 (2010).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature Rev. Cancer 8, 755–768 (2008).
Dalerba, P., Cho, R. W. & Clarke, M. F. Cancer stem cells: models and concepts. Annu. Rev. Med. 58, 267–284 (2007).
Huntly, B. J. & Gilliland, D. G. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nature Rev. Cancer 5, 311–321 (2005).
Wang, J. C. & Dick, J. E. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 15, 494–501 (2005).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Sen Banerjee, S. et al. HIF-prolyl hydroxylases and cardiovascular diseases. Toxicol. Mech. Methods 22, 347–358 (2012).
Steinhauser, M. L. et al. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature 481, 516–519 (2012).
Qu, W. et al. Synthesis of optically pure 4-fluoro-glutamines as potential metabolic imaging agents for tumors. J. Am. Chem. Soc. 133, 1122–1133 (2011).
Hosokawa, K. et al. Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell Stem Cell 6, 194–198 (2010).
Parsons, D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
Mardis, E. R. et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 361, 1058–1066 (2009).
Wise, D. R. et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc. Natl Acad. Sci. USA 108, 19611–19616 (2011).
Sasaki, M. et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 488, 656–659 (2012). Important report that demonstrates mechanistic links between an Idh1 mutation, human acute myeloid leukaemia and the induction of a leukaemic DNA methylation signature in a mouse model.
Kats, L. M. et al. Proto-oncogenic role of mutant idh2 in leukemia initiation and maintenance. Cell Stem Cell http://dx.doi.org/10.1016/j.stem.2013.12.016 (2014).
Cimmino, L., Abdel-Wahab, O., Levine, R. L. & Aifantis, I. TET family proteins and their role in stem cell differentiation and transformation. Cell Stem Cell 9, 193–204 (2011).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Dang, L., Jin, S. & Su, S. M. IDH mutations in glioma and acute myeloid leukemia. Trends Mol. Med. 16, 387–397 (2010).
Yang, H., Ye, D., Guan, K. L. & Xiong, Y. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin. Cancer Res. 18, 5562–5571 (2012).
Zhao, S. et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1α. Science 324, 261–265 (2009).
Christensen, B. C. et al. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J. Natl Cancer Inst. 103, 143–153 (2011).
Murugan, A. K., Bojdani, E. & Xing, M. Identification and functional characterization of isocitrate dehydrogenase 1 (IDH1) mutations in thyroid cancer. Biochem. Biophys. Res. Commun. 393, 555–559 (2010).
Amary, M. F. et al. Ollier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nature Genet. 43, 1262–1265 (2011).
Pansuriya, T. C. et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nature Genet. 43, 1256–1261 (2011).
Borger, D. R. et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 17, 72–79 (2012).
Wang, P. et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene 32, 3091–3100 (2013).
Gottlob, K. et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 15, 1406–1418 (2001).
Plas, D. R., Talapatra, S., Edinger, A. L., Rathmell, J. C. & Thompson, C. B. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 276, 12041–12048 (2001).
Yuneva, M., Zamboni, N., Oefner, P., Sachidanandam, R. & Lazebnik, Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell Biol. 178, 93–105 (2007).
Brunelle, J. K. et al. c-Myc sensitization to oxygen deprivation-induced cell death is dependent on Bax/Bak, but is independent of p53 and hypoxia-inducible factor-1. J. Biol. Chem. 279, 4305–4312 (2004).
Wise, D. R. et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl Acad. Sci. USA 105, 18782–18787 (2008).
Gao, P. et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765 (2009).
Wilson, A. et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 18, 2747–2763 (2004). Demonstrates that MYC controls the balance between HSC self-renewal and differentiation.
Laurenti, E., Wilson, A. & Trumpp, A. Myc's other life: stem cells and beyond. Curr. Opin. Cell Biol. 21, 844–854 (2009).
Spencer, J.A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature http://dx.doi.org/10.1038/nature13034 (2014).
Acknowledgements
The authors thank C. Lin, A. Sasaki and A. Carracedo Pérez for their comments and discussion on metabolic pathways in stem cells. T.S. is supported by the Seventh Framework Programme of the European Union under grant agreement number 306240 (SystemAge) and a Grant-in-Aid from the Japan Society for the Promotion of Science. K.I. is supported by grants from the US National Institutes of Health (NIH) (R00CA139009 and R01DK98263). The authors apologize to those whose work could not be discussed owing to space limitations.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Self-renewal
-
The capacity to propagate stem cells with a differentiation potential (potency) that is similar to that of the mother stem cell.
- Asymmetric cell division
-
One of the proposed models to explain the regulation of cell-fate decisions, which play a crucial part in stem cell self-renewal. During asymmetric division, on daughter cell remains a stem cell, while the other one becomes committed.
- Hypoxia
-
A state of reduced oxygen pressure below a certain threshold, which restricts the function of organs, tissues or cells. It has a role in regulating stem cell behaviour. A partial oxygen pressure (PO2) of <40 mm Hg in arterial blood constitutes hypoxia.
- Glycolysis
-
A metabolic pathway that generates ATP and converts one molecule of glucose into two molecules of pyruvate or lactate. Glycolysis occurs in the presence (aerobic glycolysis) or absence (anaerobic glycolysis) of oxygen.
- Long-term HSCs
-
(LT-HSCs).Haematopoietic stem cells (HSCs) that are defined functionally by their ability to mediate the long-term repopulation of all haematopoietic lineages (known as long-term repopulating activity) after transfer to lethally irradiated recipients.
- Pimonidazole
-
An effective and non-toxic exogenous 2-nitroimidazole marker for hypoxia. It forms adducts with thiol groups in proteins, peptides and amino acids specifically in hypoxic cells.
- Oxidative phosphorylation
-
(OXPHOS). The mitochondrial reactions that generate and harness energy released from the oxidation of nutrients such as pyruvate, through a proton gradient, to synthesize ATP.
- Normoxic conditions
-
Normal partial oxygen pressure (PO2) levels range from 150 mm Hg in the upper airway to ∼5 mm Hg in the retina.
- Tricarboxylic acid cycle
-
(TCA cycle). Also known as the citric acid cycle and Krebs cycle. Cyclic series of enzyme-catalysed chemical reactions that form a key part of aerobic respiration in cells. Each complete turn of the cycle generates one GTP, two CO2, one FADH2 and three NADH molecules.
- Reactive oxygen species
-
(ROS). Reactive molecules, such as hydrogen peroxide and superoxide anion. ROS form as by-products of cellular respiration and ionizing radiation, and are potentially damaging to cell structures and other molecules causing oxidative stress.
- Membrane potential
-
An important parameter of mitochondrial function that is crucial for maintaining the physiological function of the respiratory chain to generate ATP. A great loss of the membrane potential renders cells depleted of energy, followed by death.
- Metabolic reprogramming
-
Stem cell-specific programmes that may drive the dependency of stem cells on specific nutrients, such as glucose, fatty acids and Gln, as well as impose changes to the wiring of metabolic pathways to maintain stemness.
- Catabolism
-
A set of metabolic pathways that breaks down molecules (for example, nucleic acids, lipids and proteins) into smaller units (for example, nucleotides, fatty acids and amino acids) to release energy.
- Cristae
-
Folds in the inner membrane of mitochondria, which are studded with proteins, such as ATP synthase, and which increase the surface area for chemical reactions to occur (for example, cellular respiration).
- p38 MAPK
-
A member of the MAPK family that is activated by various environmental stresses and inflammatory cytokines. It is involved in processes such as cell differentiation, apoptosis and autophagy.
- p16
-
(also known as INK4A; encoded by CDKN2A). A tumour suppressor protein, which inhibits the activities of CDK4 and CDK6. This leads to cell cycle arrest and has key roles in important cellular physiological processes such as senescence and apoptosis.
- Oncogene-induced senescence
-
(OIS). A tumour suppressor mechanism that prevents the expansion of cells bearing activated oncogenes, which is caused by aberrant activation of oncoproteins and tumour suppressors.
- Pancytopenia
-
An abnormal reduction in the number of erythrocytes, leukocytes and platelets in the blood.
- Glutaminolysis
-
Biochemical reactions in which Gln is broken down to Glu, Asp, CO2, pyruvate, lactate, Ala and citrate.
- Fatty acid oxidation
-
(FAO). The dehydrogenation, hydration, oxidation and thiolysis reactions that cleave off the two-carbon acetyl-coenzyme A (CoA) from fatty acids, which then feeds into the tricarboxylic acid cycle (TCA) cycle. It also includes the generation of NADH and FADH2, which are used by the electron transport chain.
Rights and permissions
About this article
Cite this article
Ito, K., Suda, T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 15, 243–256 (2014). https://doi.org/10.1038/nrm3772
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3772
This article is cited by
-
Tumor suppressor let-7 acts as a key regulator for pluripotency gene expression in Muse cells
Cellular and Molecular Life Sciences (2024)
-
Unearthing FLVCR1a: tracing the path to a vital cellular transporter
Cellular and Molecular Life Sciences (2024)
-
SIRT6 Improves Hippocampal Neurogenesis Following Prolonged Sleep Deprivation Through Modulating Energy Metabolism in Developing rats
Molecular Neurobiology (2024)
-
Emerging roles of mitochondria in animal regeneration
Cell Regeneration (2023)
-
A state-of-the-art review on the MicroRNAs roles in hematopoietic stem cell aging and longevity
Cell Communication and Signaling (2023)