Abstract
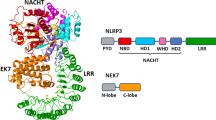
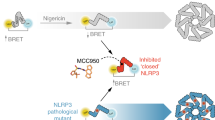
The NOD-like receptor (NLR) family, pyrin domain–containing protein 3 (NLRP3) inflammasome is a component of the inflammatory process, and its aberrant activation is pathogenic in inherited disorders such as cryopyrin-associated periodic syndrome (CAPS) and complex diseases such as multiple sclerosis, type 2 diabetes, Alzheimer's disease and atherosclerosis. We describe the development of MCC950, a potent, selective, small-molecule inhibitor of NLRP3. MCC950 blocked canonical and noncanonical NLRP3 activation at nanomolar concentrations. MCC950 specifically inhibited activation of NLRP3 but not the AIM2, NLRC4 or NLRP1 inflammasomes. MCC950 reduced interleukin-1β (IL-1β) production in vivo and attenuated the severity of experimental autoimmune encephalomyelitis (EAE), a disease model of multiple sclerosis. Furthermore, MCC950 treatment rescued neonatal lethality in a mouse model of CAPS and was active in ex vivo samples from individuals with Muckle–Wells syndrome. MCC950 is thus a potential therapeutic for NLRP3-associated syndromes, including autoinflammatory and autoimmune diseases, and a tool for further study of the NLRP3 inflammasome in human health and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wen, H., Miao, E.A. & Ting, J.P. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 39, 432–441 (2013).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Latz, E., Xiao, T.S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Lamkanfi, M. & Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Masters, S.L., Simon, A., Aksentijevich, I. & Kastner, D.L. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu. Rev. Immunol. 27, 621–668 (2009).
Wen, H., Ting, J.P. & O'Neill, L.A. A role for the NLRP3 inflammasome in metabolic diseases: did Warburg miss inflammation? Nat. Immunol. 13, 352–357 (2012).
De Nardo, D., De Nardo, C.M. & Latz, E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am. J. Pathol. 184, 42–54 (2014).
Szabo, G. & Csak, T. Inflammasomes in liver diseases. J. Hepatol. 57, 642–654 (2012).
Anders, H.J. & Muruve, D.A. The inflammasomes in kidney disease. J. Am. Soc. Nephrol. 22, 1007–1018 (2011).
Youm, Y.H. et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18, 519–532 (2013).
Masters, S.L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 (2010).
Dinarello, C.A. & van der Meer, J.W. Treating inflammation by blocking interleukin-1 in humans. Semin. Immunol. 25, 469–489 (2013).
Lamkanfi, M. et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70 (2009).
Coll, R.C., Robertson, A., Butler, M., Cooper, M. & O'Neill, L.A. The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PLoS ONE 6, e29539 (2011).
Juliana, C. et al. Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 285, 9792–9802 (2010).
He, Y. et al. 3,4-Methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J. Biol. Chem. 289, 1142–1150 (2014).
Ahn, H., Kim, J., Jeung, E.B. & Lee, G.S. Dimethyl sulfoxide inhibits NLRP3 inflammasome activation. Immunobiology 219, 315–322 (2014).
Perregaux, D.G. et al. Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J. Pharmacol. Exp. Ther. 299, 187–197 (2001).
Laliberte, R.E. et al. Glutathione S-transferase omega 1-1 is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1β post-translational processing. J. Biol. Chem. 278, 16567–16578 (2003).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Groß, O. et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36, 388–400 (2012).
Jin, C. et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc. Natl. Acad. Sci. USA 108, 14867–14872 (2011).
Netea, M.G. et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood 113, 2324–2335 (2009).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Broz, P., von Moltke, J., Jones, J.W., Vance, R.E. & Monack, D.M. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8, 471–483 (2010).
Bürckstümmer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Fernandes-Alnemri, T., Yu, J.W., Datta, P., Wu, J. & Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Roberts, T.L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009).
Kwok, B.H., Koh, B., Ndubuisi, M.I., Elofsson, M. & Crews, C.M. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IκB kinase. Chem. Biol. 8, 759–766 (2001).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Boyden, E.D. & Dietrich, W.F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38, 240–244 (2006).
Muñoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Ashcroft, F.M. ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Invest. 115, 2047–2058 (2005).
Pétrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 (2007).
Horng, T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 35, 253–261 (2014).
He, Y., Franchi, L. & Nunez, G. TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J. Immunol. 190, 334–339 (2013).
Lalor, S.J. et al. Caspase-1-processed cytokines IL-1β and IL-18 promote IL-17 production by γδ and CD4 T cells that mediate autoimmunity. J. Immunol. 186, 5738–5748 (2011).
Sutton, C., Brereton, C., Keogh, B., Mills, K.H. & Lavelle, E.C. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203, 1685–1691 (2006).
Sutton, C.E. et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009).
Gris, D. et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J. Immunol. 185, 974–981 (2010).
Brydges, S.D. et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity 30, 875–887 (2009).
Masters, S.L. et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity 37, 1009–1023 (2012).
Hoffman, H.M., Mueller, J.L., Broide, D.H., Wanderer, A.A. & Kolodner, R.D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 29, 301–305 (2001).
Gattorno, M. et al. Pattern of interleukin-1β secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 56, 3138–3148 (2007).
Lee, G.S. et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127 (2012).
Bauernfeind, F. et al. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 187, 613–617 (2011).
Brydges, S.D. et al. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J. Clin. Invest. 123, 4695–4705 (2013).
López-Castejón, G. & Pelegrin, P. Current status of inflammasome blockers as anti-inflammatory drugs. Expert Opin. Investig. Drugs 21, 995–1007 (2012).
Fautrel, B. Economic benefits of optimizing anchor therapy for rheumatoid arthritis. Rheumatology (Oxford) 51 (suppl. 4), iv21–iv26 (2012).
Urban, F.J. et al. Novel synthesis of 1-(1,2,3,5,6,7-hexahydro-s-indacen-4-yl)-3-[4-(1-hydroxy-1-methyl-ethyl)-furan-2-sulfonyl]urea, an anti-inflammatory agent. Synth. Commun. 33, 2029–2043 (2003).
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science 267, 2000–2003 (1995).
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998).
Hett, E.C. et al. Chemical genetics reveals a kinase-independent role for protein kinase R in pyroptosis. Nat. Chem. Biol. 9, 398–405 (2013).
Croker, D.E. et al. C5a2 can modulate ERK1/2 signaling in macrophages via heteromer formation with C5a1 and β-arrestin recruitment. Immunol. Cell Biol. 92, 631–639 (2014).
Sester, D.P. et al. A novel flow cytometric method to assess inflammasome formation. J. Immunol. 194, 455–462 (2015).
Westwell-Roper, C., Dunne, A., Kim, M.L., Verchere, C.B. & Masters, S.L. Activating the NLRP3 inflammasome using the amyloidogenic peptide IAPP. Methods Mol. Biol. 1040, 9–18 (2013).
Acknowledgements
We thank A. Kitanovic (German Center for Neurodegenerative Diseases) for image analysis (Fig. 3c), S. Corr (Trinity College Dublin) for providing S. typhimurium UK-1 strain and H.M. Hoffman (University of California, San Diego) for providing Nlrp3 (A350VneoR) mice. This work was supported by the following funding bodies, grants and fellowships. L.A.J.O'N. and R.C.C.: Science Foundation Ireland (G20598). D.L.K. and J.J.C.: Intramural Research Program of the National Human Genome Research Institute, US National Institutes of Health. S.C.H., C.E.S. and K.H.G.M.: Science Foundation Ireland (11/PI/1036) and (07/SRC/B1144). I.V.: Australian Research Council (FT130101215). G.N.: US National Institutes of Health (DK091191) and (DK095782). E.L.: German Research Foundation (SFB645, SFB670, SFB704, TRR57), European Research Council (ERC, InflammAct) and Excellence Cluster ImmunoSensation. S.L.M.: VESKI innovation fellowship and National Health and Medical Research Council of Australia (1032065) and (1057815). K.S.: Queensland Smart Futures Fund and Australian Research Council (FT130100361). L.A.J.O'N.: ERC Advanced Grant (E12435).
Author information
Authors and Affiliations
Contributions
R.C.C. performed and analyzed the experiments described in Figures 1b–j, 2, 3a,b and 4f–h and Supplementary Figure 6b–g; helped analyze the experiments described in Figure 5a–c and Supplementary Figure 6h,i; and wrote the manuscript. A.A.B.R. synthesized MCC950, conducted formulation for in vivo studies, determined compound pharmacokinetics, helped write the manuscript and provided advice. J.J.C. performed the experiments described in Figure 6f and Supplementary Figure 8. S.C.H. performed and analyzed the experiment described in Figure 5d–g. R.M.-P. performed the experiments described in Figure 4a,b. M.C.I. and I.V. performed the experiments described in Figure 4c–e. L.S.D. performed the experiment described in Figure 5a–c. B.G.M. and A.S. performed the experiments described in Figure 3c and Supplementary Figure 7. D.E.C. performed the experiments described in Supplementary Figure 6a. M.S.B. performed the NMR analysis described in Supplementary Figures 1, 2, 3, 4, 5 and Supplementary Table 1. M.H. performed the experiments described in Supplementary Figure 6h,i. C.E.S. helped analyze data from the experiment described in Figure 5d–g. G.N., E.L. and D.L.K. oversaw a portion of the work. K.H.G.M. conceived ideas and oversaw a portion of the work. S.L.M. performed and analyzed the experiments described in Figure 6a–e. K.S., S.L.M. and M.A.C. conceived ideas, oversaw a portion of the work, reviewed the manuscript and provided advice. L.A.J.O'N. conceived ideas, oversaw the research program and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1–5. (PDF 12971 kb)
Rights and permissions
About this article
Cite this article
Coll, R., Robertson, A., Chae, J. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21, 248–255 (2015). https://doi.org/10.1038/nm.3806
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3806
This article is cited by
-
Single-cell RNA sequencing in donor and end-stage heart failure patients identifies NLRP3 as a therapeutic target for arrhythmogenic right ventricular cardiomyopathy
BMC Medicine (2024)
-
The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases
Nature Reviews Cardiology (2024)
-
Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets
Nature Reviews Drug Discovery (2024)
-
Pathogenic NLRP3 mutants form constitutively active inflammasomes resulting in immune-metabolic limitation of IL-1β production
Nature Communications (2024)
-
A pivotal role for the IL-1β and the inflammasome in preterm labor
Scientific Reports (2024)