Abstract
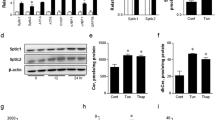
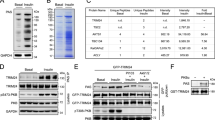
Class Ia phosphoinositide 3-kinase (PI3K), an essential mediator of the metabolic actions of insulin, is composed of a catalytic (p110α or p110β) and regulatory (p85αα, p85βα or p55α) subunit. Here we show that p85αα interacts with X-box–binding protein-1 (XBP-1), a transcriptional mediator of the unfolded protein response (UPR), in an endoplasmic reticulum (ER) stress-dependent manner. Cell lines with knockout or knockdown of p85αα show marked alterations in the UPR, including reduced ER stress–dependent accumulation of nuclear XBP-1, decreased induction of UPR target genes and increased rates of apoptosis. This is associated with a decreased activation of inositol-requiring protein-1α (IRE1α) and activating transcription factor-6αα (ATF6α). Mice with deletion of p85α in liver (L-Pik3r1−/−) show a similar attenuated UPR after tunicamycin administration, leading to an increased inflammatory response. Thus, p85αα forms a previously unrecognized link between the PI3K pathway, which is central to insulin action, and the regulation of the cellular response to ER stress, a state that when unresolved leads to insulin resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gotto, A.M. Jr. et al. The metabolic syndrome: a call to action. Coron. Artery Dis. 17, 77–80 (2006).
Ford, E.S., Giles, W.H. & Mokdad, A.H. Increasing prevalence of the metabolic syndrome among US adults. Diabetes Care 27, 2444–2449 (2004).
Dandona, P., Aljada, A. & Bandyopadhyay, A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 25, 4–7 (2004).
Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Vanhaesebroeck, B., Stein, R.C. & Waterfield, M.D. The study of phosphoinositide 3-kinase function. Cancer Surv. 27, 249–270 (1996).
Cheatham, B. et al. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis and glucose transporter translocation. Mol. Cell. Biol. 14, 4902–4911 (1994).
Sharma, P.M. et al. Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J. Biol. Chem. [In Process Citation] 273, 18528–18537 (1998).
Cusi, K. et al. Insulin resistance differentially affects the PI 3-kinase– and MAP kinase–mediated signaling in human muscle. J. Clin. Invest. 105, 311–320 (2000).
Heydrick, S.J., Gautier, N., Olichon-Berthe, C., Van Obberghen, E. & Le Marchand-Brustel, Y Early alteration of insulin stimulation of PI 3-kinase in muscle and adipocyte from gold thioglucose obese mice. Am. J. Physiol. 268, E604–E612 (1995).
Bandyopadhyay, G.K., Yu, J.G., Ofrecio, J. & Olefsky, J.M. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes 54, 2351–2359 (2005).
Ni, M. & Lee, A.S. ER chaperones in mammalian development and human diseases. FEBS Lett. 581, 3641–3651 (2007).
Dobson, C.M. Principles of protein folding, misfolding and aggregation. Semin. Cell Dev. Biol. 15, 3–16 (2004).
Schröder, M. & Kaufman, R. ER stress and the unfolded protein response. Mutat. Res. 569, 29–63 (2005).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Elouil, H. et al. Acute nutrient regulation of the unfolded protein response and integrated stress response in cultured rat pancreatic islets. Diabetologia 50, 1442–1452 (2007).
Lee, A.S. Mammalian stress response: induction of the glucose-regulated protein family. Curr. Opin. Cell Biol. 4, 267–273 (1992).
He, B. Viruses, endoplasmic reticulum stress and interferon responses. Cell Death Differ. 13, 393–403 (2006).
Boden, G. et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57, 2438–2444 (2008).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Eizirik, D.L., Cardozo, A.K. & Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29, 42–61 (2008).
Gregor, M.F. et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58, 693–700 (2009).
Ozawa, K. et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 54, 657–663 (2005).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Tanti, J.F. et al. Alteration in insulin action: role of IRS-1 serine phosphorylation in the retroregulation of insulin signalling. Ann. Endocrinol. (Paris) 65, 43–48 (2004).
Gao, Z. et al. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol. Endocrinol. 18, 2024–2034 (2004).
Herschkovitz, A. et al. Common inhibitory serine sites phosphorylated by IRS-1 kinases, triggered by insulin and inducers of insulin resistance. J. Biol. Chem. 282, 18018–18027 (2007).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Ron, D. & Hubbard, S.R. How IRE1 reacts to ER stress. Cell 132, 24–26 (2008).
Ueki, K. et al. Positive and negative roles of p85α and p85β regulatory subunits of phosphoinositide 3-kinase in insulin signaling. J. Biol. Chem. 278, 48453–48466 (2003).
Terauchi, Y. et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85α subunit of phosphoinositide 3-kinase. Nat. Genet. 21, 230–235 (1999).
Taniguchi, C.M. et al. The p85α regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase–mediated insulin resistance. Mol. Cell. Biol. 27, 2830–2840 (2007).
Forler, D. et al. An efficient protein complex purification method for functional proteomics in higher eukaryotes. Nat. Biotechnol. 21, 89–92 (2003).
Rigaut, G. et al. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17, 1030–1032 (1999).
Tirosh, B., Iwakoshi, N.N., Glimcher, L.H. & Ploegh, H.L. Rapid turnover of unspliced Xbp-1 as a factor that modulates the unfolded protein response. J. Biol. Chem. 281, 5852–5860 (2006).
Yoshida, H., Oku, M., Suzuki, M. & Mori, K. pXBP1U encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1S in mammalian ER stress response. J. Cell Biol. 172, 565–575 (2006).
Fonseca, S.G., Lipson, K.L. & Urano, F. Endoplasmic reticulum stress signaling in pancreatic beta-cells. Antioxid. Redox Signal. 9, 2335–2344 (2007).
Harding, H.P. & Ron, D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes 51 Suppl 3, S455–S461 (2002).
Eizirik, D.L., Cardozo, A.K. & Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29, 42–61 (2008).
Nakatani, Y. et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J. Biol. Chem. 280, 847–851 (2005).
Shoelson, S.E., Lee, J. & Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801 (2006).
Shepherd, P.R., Withers, D.J. & Siddle, K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem. J. 333, 471–490 (1998).
Duronio, V., Scheid, M.P. & Ettinger, S. Downstream signalling events regulated by phosphatidylinositol 3-kinase activity. Cell. Signal. 10, 233–239 (1998).
Barbour, L.A. et al. Human placental growth hormone increases expression of the p85 regulatory unit of phosphatidylinositol 3-kinase and triggers severe insulin resistance in skeletal muscle. Endocrinology 145, 1144–1150 (2004).
Khalfallah, Y., Sassolas, G., Borson-Chazot, F., Vega, N. & Vidal, H. Expression of insulin target genes in skeletal muscle and adipose tissue in adult patients with growth hormone deficiency: effect of one year recombinant human growth hormone therapy. J. Endocrinol. 171, 285–292 (2001).
Barbour, L.A. et al. Human placental growth hormone increases expression of the p85 regulatory unit of phosphatidylinositol 3-kinase and triggers severe insulin resistance in skeletal muscle. Endocrinology 145, 1144–1150 (2004).
Luo, J. & Cantley, L.C. The negative regulation of phosphoinositide 3-kinase signaling by p85 and it's implication in cancer. Cell Cycle 4, 1309–1312 (2005).
Yeates, L.C., Gallegos, A., Kozikowski, A.P. & Powis, G. Down regulation of the expression of the p110, p85 and p55 subunits of phosphatidylinositol 3-kinase during colon cancer cell anchorage-independent growth. Anticancer Res. 19, 4171–4176 (1999).
Fang, D. & Liu, Y.C. Proteolysis-independent regulation of PI3K by Cbl-b–mediated ubiquitination in T cells. Nat. Immunol. 2, 870–875 (2001).
García, Z. et al. A PI3K activity–independent function of p85 regulatory subunit in control of mammalian cytokinesis. EMBO J. 25, 4740–4751 (2006).
Park, S.W., Zhou, Y., Lee, J., Lu, A., Sun, C., Chung, J., Ueki, K. & Ozcan, U. Regulatory subunits of PI3K, p85α and p85β, interact with XBP1 and increase its nuclear translocation. Nat. Med. advance online publication, doi:10.1038/nm.2099 (28 March 2010).
Yamamoto, K. et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell 13, 365–376 (2007).
Mauvais-Jarvis, F. et al. Reduced expression of the murine p85α subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes. J. Clin. Invest. 109, 141–149 (2002).
Chen, D. et al. p50α/p55α phosphoinositide 3-kinase knockout mice exhibit enhanced insulin sensitivity. Mol. Cell. Biol. 24, 320–329 (2004).
Brachmann, S.M., Ueki, K., Engelman, J.A., Kahn, R.C. & Cantley, L.C. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol. Cell. Biol. 25, 1596–1607 (2005).
Wellen, K.E. & Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 (2005).
Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Kerouz, N.J., Hörsch, D., Pons, S. & Kahn, C.R. Differential regulation of insulin receptor substrates-1 and -2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse. J. Clin. Invest. 100, 3164–3172 (1997).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Brüning, J.C., Winnay, J., Cheatham, B. & Kahn, C.R. Mol. Cell Biol. 17, 1513–1521 (1997).
Marciniak, S.J. et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 (2004).
Acknowledgements
We would like to thank D. Ron (New York University School of Medicine) for providing the Flag–XBP-1u and Flag–XBP-1s expression plasmids. This work was supported by US National Institutes of Health grant DK55545 and National Institutes of Health training grant DK07260-30, as well as Core laboratory support from the Joslin Diabetes and Endocrine Research Center grant DK36836. We would also like to thank U. Ozcan for useful advice and discussion and S. Green and S. Flaherty for assistance in preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
J.N.W. came up with the hypothesis, designed and performed the experiments, analyzed the data and wrote the manuscript. J.B. and M.A.M. designed and performed the experiments and analyzed the data. K.U. came up with the hypothesis and generated reagents. C.R.K. came up with the hypothesis, helped analyze the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 1412 kb)
Rights and permissions
About this article
Cite this article
Winnay, J., Boucher, J., Mori, M. et al. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box–binding protein-1 to modulate the unfolded protein response. Nat Med 16, 438–445 (2010). https://doi.org/10.1038/nm.2121
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2121
This article is cited by
-
Exploring mechanisms underlying diabetes comorbidities and strategies to prevent vascular complications
Diabetology International (2024)
-
The activation of spliced X-box binding protein 1 by isorhynchophylline therapy improves diabetic encephalopathy
Cell Biology and Toxicology (2023)
-
Tripartite motif 25 ameliorates doxorubicin-induced cardiotoxicity by degrading p85α
Cell Death & Disease (2022)
-
Live and let die: signaling AKTivation and UPRegulation dynamics in SARS-CoVs infection and cancer
Cell Death & Disease (2022)
-
Nuclear translocation of p85β promotes tumorigenesis of PIK3CA helical domain mutant cancer
Nature Communications (2022)