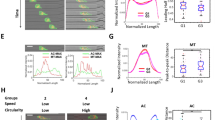
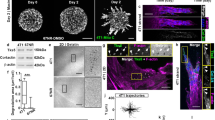
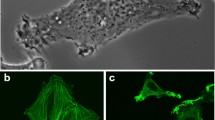
Abstract
Here we use intravital imaging to demonstrate a reversible transition to a motile state as breast cancer cells spread. Imaging primary tumours revealed heterogeneity in cell morphology and motility. Two distinct modes of motility were observed: collective and single-celled. By monitoring the localization of Smad2 and the activity of a TGFβ-dependent reporter gene during breast cancer cell dissemination, we demonstrate that TGFβ signalling is transiently and locally activated in motile single cells. TGFβ1 switches cells from cohesive to single cell motility through a transcriptional program involving Smad4, EGFR, Nedd9, M-RIP, FARP and RhoC. Blockade of TGFβ signalling prevented cells moving singly in vivo but did not inhibit cells moving collectively. Cells restricted to collective invasion were capable of lymphatic invasion but not blood-borne metastasis. Constitutive TGFβ signalling promoted single cell motility and intravasation but reduced subsequent growth in the lungs. Thus, transient TGFβ signalling is essential for blood-borne metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nature Rev. Cancer 2, 563–572 (2002).
Sahai, E. Illuminating the metastatic process. Nature Rev. Cancer 7, 737–749 (2007).
Friedl, P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr. Opin. Cell Biol. 16, 14–23 (2004).
Leivonen, S. K. & Kahari, V. M. Transforming growth factor-β signaling in cancer invasion and metastasis. Int. J. Cancer 121, 2119–2124 (2007).
Lesko, E. & Majka, M. The biological role of HGF-MET axis in tumor growth and development of metastasis. Front. Biosci. 13, 1271–1280 (2008).
Wells, A. Tumor invasion: role of growth factor-induced cell motility. Adv. Cancer Res 78, 31–101 (2000).
Levy, L. & Hill, C. S. Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 17, 41–58 (2006).
Nicholson, R. I., Gee, J. M. & Harper, M. E. EGFR and cancer prognosis. Eur. J. Cancer 37 Suppl 4, S9–S15 (2001).
Fox, S. B. & Harris, A. L. The epidermal growth factor receptor in breast cancer. J. Mammary Gland Biol. Neoplasia 2, 131–141 (1997).
Siegel, P. M. & Massague, J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Naturet Rev. Cancer 3, 807–821 (2003).
Donovan, J. & Slingerland, J. Transforming growth factor-β and breast cancer: Cell cycle arrest by transforming growth factor-beta and its disruption in cancer. Breast Cancer Res. 2, 116–124 (2000).
Wakefield, L. M. & Roberts, A. B. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 12, 22–29 (2002).
Jakowlew, S. B. Transforming growth factor-β in cancer and metastasis. Cancer Metastasis Rev. 25, 435–457 (2006).
Zavadil, J. & Bottinger, E. P. TGF-β and epithelial-to-mesenchymal transitions. Oncogene 24, 5764–5774 (2005).
Vincent-Salomon, A. & Thiery, J. P. Host microenvironment in breast cancer development: epithelial-mesenchymal transition in breast cancer development. Breast Cancer Res. 5, 101–106 (2003).
Kokkinos, M. I. et al. Vimentin and epithelial-mesenchymal transition in human breast cancer--observations in vitro and in vivo. Cells Tissues Organs 185, 191–203 (2007).
Condeelis, J. & Segall, J. E. Intravital imaging of cell movement in tumours. Nature Rev. Cancer 3, 921–930 (2003).
Wyckoff, J. B., Jones, J. G., Condeelis, J. S. & Segall, J. E. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 60, 2504–2511 (2000).
ten Dijke, P. & Hill, C. S. New insights into TGF-β-Smad signalling. Trends Biochem. Sci. 29, 265–273 (2004).
Wyckoff, J. B. et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67, 2649–2656 (2007).
Philippar, U. et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev. Cell 15, 813–828 (2008).
Sahai, E. et al. Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol. 5, 14 (2005).
Xue, C. et al. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res. 66, 192–197 (2006).
Dennler, S. et al. Direct binding of Smad3 and Smad4 to critical TGF β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 17, 3091–3100 (1998).
Schmierer, B. & Hill, C. S. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor β-dependent nuclear accumulation of Smads. Mol. Cell Biol. 25, 9845–9858 (2005).
Shaner, N. C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nature Biotechnol. 22, 1567–1572 (2004).
Mi, Z. et al. Differential osteopontin expression in phenotypically distinct subclones of murine breast cancer cells mediates metastatic behavior. J. Biol. Chem. 279, 46659–46667 (2004).
Wyckoff, J. B., Pinner, S. E., Gschmeissner, S., Condeelis, J. S. & Sahai, E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 16, 1515–1523 (2006).
Padua, D. et al. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133, 66–77 (2008).
Welch, D. R., Fabra, A. & Nakajima, M. Transforming growth factor β stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc. Natl Acad. Sci. USA 87, 7678–7682 (1990).
Goswami, S. et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 65, 5278–5283 (2005).
Gupta, G. P. et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446, 765–770 (2007).
Kang, Y. et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl Acad. Sci. USA 102, 13909–13914 (2005).
Nicolas, F. J., De Bosscher, K., Schmierer, B. & Hill, C. S. Analysis of Smad nucleocytoplasmic shuttling in living cells. J. Cell Sci. 117, 4113–4125 (2004).
Gilles, C. et al. Transactivation of vimentin by β-catenin in human breast cancer cells. Cancer Res. 63, 2658–2664 (2003).
Wong, C. et al. Smad3-Smad4 and AP-1 complexes synergize in transcriptional activation of the c-Jun promoter by transforming growth factor β. Mol. Cell Biol. 19, 1821–1830 (1999).
Acknowledgements
We thank lab colleagues for advice and comments on this project. We thank members of the LRI FACS laboratory, Clare Watkins and members of the LRI Biological Resources Unit, the Paterson Institute for Cancer Research microarray facility, the LRI Bioinformatics team, Mike Howell of the LRI Developmental Signalling laboratory and Debbie Aubyn of the LRI microscopy laboratory for technical assistance. This work was funded by the Breast Cancer Campaign project grant 12May05 and CRUK.
Author information
Authors and Affiliations
Contributions
S.G. generated most of the data in Figs 2, 3, 4, 5 and 7 with some assistance from E.S. S.G. and E.S. made equal contributions to Fig. 8 with assistance from C.S.M.; E.S. generated most of the data in Figs 1 and 6 with assistance from S.G. and S.H.; L.J.J. provided clinical material used in Supplementary Information figures; S.G. generated data shown in Supplementary Information Figs 3, 4, 5, 6, 7, 8; S.H. and C.S.M. assisted with Supplementary Information Figs 7 and 8, respectively; E.S. generated data in Supplementary Information Figs 1, 2, 6 and 9; C.S.H. provided reagents, expertise and intellectual input. S.G. and E.S. designed the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2708 kb)
Supplementary Information
Supplementary Movie 1 (AVI 3506 kb)
Supplementary Information
Supplementary Movie 2 (AVI 620 kb)
Supplementary Information
Supplementary Movie 3 (AVI 1030 kb)
Supplementary Information
Supplementary Movie 4 (AVI 1378 kb)
Supplementary Information
Supplementary Movie 5 (AVI 1928 kb)
Supplementary Information
Supplementary Movie 6 (AVI 470 kb)
Supplementary Information
Supplementary Movie 7 (AVI 680 kb)
Supplementary Information
Supplementary Movie 8 (AVI 2097 kb)
Supplementary Information
Supplementary Movie 9 (AVI 2054 kb)
Supplementary Information
Supplementary Movie 10 (AVI 691 kb)
Supplementary Information
Supplementary Movie 11 (AVI 601 kb)
Supplementary Information
Supplementary Movie 12 (AVI 2810 kb)
Supplementary Information
Supplementary Movie 13 (AVI 2741 kb)
Supplementary Information
Supplementary Movie 14 (AVI 3051 kb)
Supplementary Information
Supplementary Movie 15 (AVI 2074 kb)
Supplementary Information
Supplementary Movie 16 (AVI 1238 kb)
Supplementary Information
Supplementary Movie 17 (AVI 1165 kb)
Supplementary Information
Supplementary Movie 18 (AVI 319 kb)
Rights and permissions
About this article
Cite this article
Giampieri, S., Manning, C., Hooper, S. et al. Localized and reversible TGFβ signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 11, 1287–1296 (2009). https://doi.org/10.1038/ncb1973
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1973
This article is cited by
-
A model for the dissemination of circulating tumour cell clusters involving platelet recruitment and a plastic switch between cooperative and individual behaviours
BMC Ecology and Evolution (2023)
-
Intravital imaging to study cancer progression and metastasis
Nature Reviews Cancer (2023)
-
Prediction of cell migration potential on human breast cancer cells treated with Albizia lebbeck ethanolic extract using extreme machine learning
Scientific Reports (2023)
-
Proteogenomics decodes the evolution of human ipsilateral breast cancer
Communications Biology (2023)
-
The dynamic tumor–stromal crosstalk: implications of ‘stromal-hot’ tumors in the process of epithelial–mesenchymal transition in breast cancer
Molecular Biology Reports (2023)