Abstract
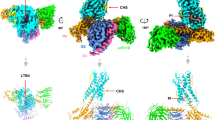
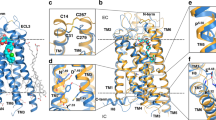
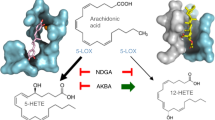
Cysteinyl leukotrienes are key mediators in inflammation and have an important role in acute and chronic inflammatory diseases of the cardiovascular and respiratory systems, in particular bronchial asthma. In the biosynthesis of cysteinyl leukotrienes, conversion of arachidonic acid forms the unstable epoxide leukotriene A4 (LTA4). This intermediate is conjugated with glutathione (GSH) to produce leukotriene C4 (LTC4) in a reaction catalysed by LTC4 synthase1: this reaction is the key step in cysteinyl leukotriene formation. Here we present the crystal structure of the human LTC4 synthase in its apo and GSH-complexed forms to 2.00 and 2.15 Å resolution, respectively. The structure reveals a homotrimer, where each monomer is composed of four transmembrane segments. The structure of the enzyme in complex with substrate reveals that the active site enforces a horseshoe-shaped conformation on GSH, and effectively positions the thiol group for activation by a nearby arginine at the membrane–enzyme interface. In addition, the structure provides a model for how the ω-end of the lipophilic co-substrate is pinned at one end of a hydrophobic cleft, providing a molecular ‘ruler’ to align the reactive epoxide at the thiol of glutathione. This provides new structural insights into the mechanism of LTC4 formation, and also suggests that the observed binding and activation of GSH might be common for a family of homologous proteins important for inflammatory and detoxification responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lam, B. K. & Austen, K. F. Leukotriene C-4 synthase: A pivotal enzyme in cellular biosynthesis of the cysteinyl leukotrienes. Prostaglandins Other Lipid Mediat. 68-69, 511–520 (2002)
Samuelsson, B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science 220, 568–575 (1983)
Funk, C. D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 294, 1871–1875 (2001)
Samuelsson, B., Dahlén, S.-E., Lindgren, J.-Å., Rouzer, C. A. & Serhan, C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 237, 1171–1176 (1987)
Hanna, C. J., Bach, M. K., Pare, P. D. & Schellenberg, R. R. Slow-reacting substances (leukotrienes) contract human airway and pulmonary vascular smooth-muscle in vitro. Nature 290, 343–344 (1981)
Dahlén, S.-E., Hedqvist, P., Hammarström, S. & Samuelsson, B. Leukotrienes are potent constrictors of human bronchi. Nature 288, 484–486 (1980)
Dahlén, S.-E. et al. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: In vivo effects with relevance to the acute inflammatory response. Proc. Natl Acad. Sci. USA 78, 3887–3891 (1981)
Weiss, J. W. et al. Bronchoconstrictor effects of leukotriene-C in humans. Science 216, 196–198 (1982)
Drazen, J. F., Israel, E. & O’Byrne, P. Treatment of asthma with drugs modifying the leukotriene pathway. N. Engl. J. Med. 340, 197–206 (1999)
Bisgaard, H. Leukotriene modifiers in pediatric asthma management. Pediatrics 107, 381–390 (2001)
Jakobsson, P. J., Morgenstern, R., Mancini, J., Ford-Hutchinson, A. & Persson, B. Common structural features of MAPEG — a widespread superfamily of membrane associated proteins with highly divergent functions in eicosanoid and glutathione metabolism. Protein Sci. 8, 689–692 (1999)
Morgenstern, R., Svensson, R., Bernat, B. A. & Armstrong, R. N. Kinetic analysis of the slow ionization of glutathione by microsomal glutathione transferase MGST1. Biochemistry (Mosc.) 40, 3378–3384 (2001)
Corey, E. J. et al. Stereospecific total synthesis of a “slow reacting substance” of anaphylaxis, leukotriene C4. J. Am. Chem. Soc. 102, 1436–1439 (1980)
Holm, P. J. et al. Structural basis for detoxification and oxidative stress protection in membranes. J. Mol. Biol. 360, 934–945 (2006)
Izumi, T. et al. Solubilization and partial purification of leukotriene C4 synthase from guinea-pig lung: A microsomal enzyme with high specificity towards 5,6-epoxide leukotriene A4. Biochim. Biophys. Acta 959, 305–315 (1988)
Yoshimoto, T., Soberman, R. J., Spur, B. & Austen, K. F. Properties of highly purified leukotriene C4 synthase of guinea pig lung. J. Clin. Invest. 81, 866–871 (1988)
Leslie, A. G. W. Recent changes to the MOSFLM package for processing film and image plate data. Joint CCP4 + ESF-EAMCB Newsl. Protein Crystallogr. 26, 27 (1992)
Collaborative. Computational Project, Number 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 (1993)
Uson, I. & Sheldrick, G. M. Advances in direct methods for protein crystallography. Curr. Opin. Struct. Biol. 9, 643–648 (1999)
de La Fortelle, E. & Bricogne, G. Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol. 276, 472–494 (1997)
Abrahams, J. P. & Leslie, A. G. W. Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr. D 52, 30–42 (1996)
Perrakis, A., Morris, R. & Lamzin, V. S. Automated protein model building combined with iterative structure refinement. Nature Struct. Biol. 6, 458–463 (1999)
Emsley, P. & Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
McCoy, A. J., Grosse-Kunstleve, R. W., Storoni, L. C. & Read, R. J. Likelihood-enhanced fast translation functions. Acta Crystallogr. D 61, 458–464 (2005)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
DeLano, W. L. The PyMOL Molecular Graphics System. (DeLano Scientific, Palo Alto, 2002)
Wallace, A. C., Laskowski, R. A. & Thornton, J. M. Ligplot — a program to generate schematic diagrams of protein ligand interactions. Protein Eng. 8, 127–134 (1995)
Acknowledgements
This work was supported by the Swedish Research Council, the Swedish Cancer Society, the Göran Gustafsson foundation, the Knut och Alice Wallenberg foundation, the EU integrated projects E-MEP and EICOSANOX. This Letter reflects only the authors’ views and the European Commission is not liable for any use that may be made of the information herein. We also thank staff at ESRF, BESSY, SLS and MAXLAB for beam time and assistance during data collection. We are grateful to T. Cornvik for help with artwork and to T. Bergman for help with mass spectrometry analysis.
Author Contributions A.W., T.H. and J.Z.H. cloned, expressed and purified LTC4 synthase. Further purification and characterization of LTC4 synthase in various detergents was performed by A.W., D.M.M. and E.O. and was supervised by S.E. D.M.M. crystallized LTC4 synthase and solved the structure together with A.A.M. and A.K. Crystallographic work was performed by D.M.M. under supervision of A.K. and P.N. Extensive crystal and derivative screening was conducted by D.M.M., A.K. and D.N. Overall project management and strategy was performed by J.Z.H. and P.N. Writing of the manuscript was performed jointly by P.N., J.Z.H., S.E. and D.M.M. All authors discussed the results and commented on the manuscript.
Coordinates and structure factors for LTC4 synthase in its apo form and in complex with GSH have been deposited in the Protein Data Bank with accession codes 2UUI and 2UUH, respectively.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1–S5 and Supplementary Table S1. The Supplementary Figures illustrate a schematic drawing of the reaction catalyzed by LTC4 synthase, the metal coordination and crystal packing, a stereo view of the bound substrate, a lig plot diagram of the bound substrate and finally a structure based sequence alignment of the MAPEG family. A supplementary Table is included with crystallographic statistics. (PDF 1645 kb)
Rights and permissions
About this article
Cite this article
Molina, D., Wetterholm, A., Kohl, A. et al. Structural basis for synthesis of inflammatory mediators by human leukotriene C4 synthase. Nature 448, 613–616 (2007). https://doi.org/10.1038/nature06009
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06009
This article is cited by
-
Crystal structures of human MGST2 reveal synchronized conformational changes regulating catalysis
Nature Communications (2021)
-
The enzymology of human eicosanoid pathways: the lipoxygenase branches
Molecular Biology Reports (2020)
-
Label-Free Quantitative Proteomic Reveals Differentially Expressed Proteins in Aeromonas-Immunostimulated Leukocytes of Lampetra japonica
Current Microbiology (2018)
-
Dead-end complex, lipid interactions and catalytic mechanism of microsomal glutathione transferase 1, an electron crystallography and mutagenesis investigation
Scientific Reports (2017)
-
Overexpression of membrane proteins from higher eukaryotes in yeasts
Applied Microbiology and Biotechnology (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.