Abstract
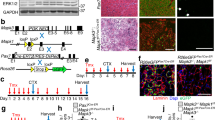
Aging skeletal muscles suffer a steady decline in mass and functional performance, and compromised muscle integrity as fibrotic invasions replace contractile tissue, accompanied by a characteristic loss in the fastest, most powerful muscle fibers1,2. The same programmed deficits in muscle structure and function are found in numerous neurodegenerative syndromes and disease-related cachexia3. We have generated a model of persistent, functional myocyte hypertrophy using a tissue-restricted transgene encoding a locally acting isoform of insulin-like growth factor-1 that is expressed in skeletal muscle (mIgf-1). Transgenic embryos developed normally, and postnatal increases in muscle mass and strength were not accompanied by the additional pathological changes seen in other Igf-1 transgenic models. Expression of GATA-2, a transcription factor normally undetected in skeletal muscle, marked hypertrophic myocytes that escaped age-related muscle atrophy and retained the proliferative response to muscle injury characteristic of younger animals. The preservation of muscle architecture and age-independent regenerative capacity through localized mIgf-1 transgene expression suggests clinical strategies for the treatment of age or disease-related muscle frailty.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Brooks, S. & Faulkner, J. Contractile properties of skeletal muscles from young, adult and aged mice. J. Physiol. 404, 71–82 (1988).
Musaro, A. et al. Enhanced expression of myogenic regulatory factors in aging skeletal muscle. Exp. Cell Res. 221, 241–248 (1995).
Nelson, K. The cancer anorexia-cachexia syndrome. Semin. Oncol. 27, 64–68 (2000).
Florini, J., Ewton, D. & Coolican, S. Growth hormone and the insulin-like growth factor system in myogenesis. Endocrine Rev. 17, 481–517 (1996).
Stewart, C. & Rotwein, P. Growth, differentation, and survival: multiple physiological functions for insulin-like growth factors. Physiol. Rev. 76, 1005–1026 (1996).
Sjogren, K. et al. Liver-derived IGF-1 is the principal source of IGF-1 in blood but is not required for postnatal body growth in mice. Proc. Natl. Acad. Sci. USA 96, 7088–7092 (1999).
Rosenthal, S. & Cheng, Z.Q. Opposing early and late effects of insulin-like growth factor I on differentiation and the cell cycle regulatory retinoblastoma protein in skeletal myoblasts. Proc. Natl. Acad. Sci. USA 92, 10307–10311 (1995).
Engert, J., Berglund, E.B. & Rosenthal, N. Proliferation precedes differentiation in IGF-1 stimulated myogenesis. J. Cell Biol. 135, 431–440 (1996).
Mathews, L. et al. Growth enhancement of transgenic mice expressing human insulin-like growth factor-I. Endocrinology 123, 2827–2833 (1988).
Coleman, M. et al. Myogenic vector expression of insulin-like growth factor I stimulate myocyte differentiation and myofiber hypertrophy in transgenic mice. J. Biol. Chem. 270, 12109–12116 (1995).
Reiss, K. et al. Overexpression of insulin-like growth factor-I in the heart is coupled with myocyte proliferation in transgenic mice. Proc. Natl. Acad. Sci. USA 93, 8630–8635 (1996).
Delaughter, M.C., Taffet, G.E., Fiorotto, M.L., Entman, M.L. & Schwartz, R.J. Local insulin-like growth factor I expression induces physiologic, then pathologic cardiac hypertrophy in transgenic mice. FASEB J. 13, 1923–1929 (1999).
Adamo, M. et al. Structure, expression and regulation of the IGF-1 gene. Adv. Exp. Med. Biol. 343, 1–11 (1993).
Grieshammer, U., Sassoon, D. & Rosenthal, N. A transgene target for positional regulators marks early rostrocaudal specification of myogenic lineages. Cell 69, 79–93 (1992).
Musaro, A. & Rosenthal, N. Maturation of the myogenic program is induced by post-mitotic expression of IGF-1. Mol. Cell. Biol. 19, 3115–3124 (1999).
Musaro, A., McKullagh, K.J.A., Naya, F.J., Olson, E.N. & Rosenthal, N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in assocation with GATA-2 and NF-ATc1. Nature 400, 581–585 (1999).
Semsarian, C. et al. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature 400, 576–581 (1999).
Molkentin, J. et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93, 215–228 (1998).
Lim, H. et al. Reversal of cardiac hypertrophy in transgenic disease models by calcineurin inhibition. J. Mol. Cell. Cardiol. 32, 697–709 (2000).
Chin, E. et al. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 12, 2499–2509 (1998).
Naya, F. et al. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem. 275, 4545–4548 (2000).
Bigard, X. et al. Calcineurin co-regulates contractile and metabolic components of slow muscle phenotype. J. Biol. Chem. 275, 19653–19660 (2000).
Abbott, K.L., Friday, B.B., Thaloor, D., Murphy, T.J. & Pavlath, G.K. Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol. Biol. Cell 9, 2905–2916 (1998).
Passier, R. et al. CaM kinase singaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J. Clin. Invest. 105, 1395–1406 (2000).
Lescaudron, L., Creuzet, S.E., Li, Z., Paulin, D. & Fontaine-Perus, J. Desmin-lacZ transgene expression and regeneration within skeletal muscle transplants. J. Muscle Res. Cell. Motil. 18, 631–641 (1997).
Barton-Davis, E., Shoturma, D.I., Musaro, A., Rosenthal, N. & Sweeney, H.L. Viral mediated expression of IGF-1 blocks the aging-related loss of skeletal muscle function. Proc. Natl. Acad. Sci. USA 95, 15603–15607 (1998).
Barton-Davis, E.R., Shoturma, D.I. & Sweeney, H.L. Contribution of satellite cells to IGF-1 induced hypertrophy of skeletal muscle. Acta Physiol. Scand. 167, 301–305 (1999).
LeRoith, D. & Roberts, C.T., Jr. At the cutting edge. Insulin-like growth factor I (IGF-1): a molecular basis for endocrine versus local action. Mol. Cell. Endocrinol. 77, C57–C61 (1991).
McKoy, G. et al. Expression of insulin growth factor-I splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J. Physiol. 516, 583–592 (1999).
Acknowledgements
We thank J. Gonzales, E. Slonimsky and S. Zaltsman for generation and characterization of transgenic mouse lines and histological analysis; F. Depreux, C. Neville, L. Tsao and other members of the Rosenthal lab for advice and discussion; and G. Cossu for critical comments on this manuscript. A.M. was supported by a Research Development Award from the Muscular Dystrophy Association. This work was funded by grants to N.R. and L.S. from the National Institute on Aging and the Muscular Dystrophy Association, and by a grant to N.R. from NASA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Musarò, A., McCullagh, K., Paul, A. et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27, 195–200 (2001). https://doi.org/10.1038/84839
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/84839
This article is cited by
-
Cellular senescence contributes to mechanical ventilation-induced diaphragm dysfunction by upregulating p53 signalling pathways
BMC Pulmonary Medicine (2023)
-
Soybean isoflavones potentially prevent sarcopenia: a systematic review
Journal of Ethnic Foods (2023)
-
Skeletal muscle overexpression of sAnk1.5 in transgenic mice does not predispose to type 2 diabetes
Scientific Reports (2023)
-
Myostatin-mediated regulation of skeletal muscle damage post-acute Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus L.)
Fish Physiology and Biochemistry (2023)
-
The myokine Fibcd1 is an endogenous determinant of myofiber size and mitigates cancer-induced myofiber atrophy
Nature Communications (2022)