Abstract
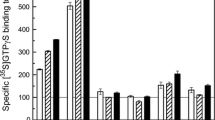
1. The bark of the root and stem of various Magnolia species has been used in Traditional Chinese Medicine to treat a variety of disorders including anxiety and nervous disturbances. The biphenolic compounds honokiol (H) and magnolol (M), the main components of the Chinese medicinal plant Magnolia officinalis, interact with GABAA receptors in rat brain in vitro. We compared the effects of H and M on [3H]muscimol (MUS) and [3H]flunitrazepam (FNM) binding using EDTA/water dialyzed rat brain membranes in a buffer containing 150 mM NaCl plus 5 mM Tris-HCl, pH 7.5 as well as [35S]t-butylbicyclophosphorothionate (TBPS) in 200 mM KBr plus 5 mM Tris-HCl, pH 7.5. H and M had similar enhancing effects on [3H]MUS as well as on [3H]FNM binding to rat brain membrane preparations, but H was 2.5 to 5.2 times more potent than M. 2. [ 3 H]FNM binding. GABA alone almost doubled [3H]FNM binding with EC50 = 450 nM and 200 nM using forebrain and cerebellar membranes, respectively. In the presence of 5 μM H or M the EC50 values for GABA were decreased to 79 and 89 nM, respectively, using forebrain, and 39 and 78 nM, using cerebellar membranes. H and M potently enhanced the potentiating effect of 200 nM GABA on [3H]FNM binding with EC50 values of 0.61 μM and 1.6 μM using forebrain membranes, with maximal enhancements of 33 and 47%, respectively. Using cerebellar membranes, the corresponding values were 0.25 and 1.1 μM, and 22 and 34%. 3. [ 3 H]MUS binding. H and M increased [3H]MUS binding to whole forebrain membranes about 3-fold with EC50 values of 6.0 and 15 μM. Using cerebellar membranes, H and M increased [3H]MUS binding ~68% with EC50 values of 2.3 and 12 μM, respectively. Scatchard analysis revealed that the enhancements of [3H]MUS binding were due primarily to increases in the number of binding sites (Bmax values) with no effect on the high affinity binding constants (Kd values). The enhancing effect of H and M were not additive. 4. [ 35 S]TBPS binding. H and M displaced [35S]TBPS binding from sites on whole rat forebrain membranes with IC50 values of 7.8 and 6.0 μM, respectively. Using cerebellar membranes, the corresponding IC50 values were 5.3 and 4.8 μM. These inhibitory effects were reversed by the potent GABAA receptor blocker R5135 (10 nM), suggesting that H and M allosterically increase the affinity of GABAA receptors for GABA and MUS by binding to sites in GABAA receptor complexes. 5. Two monophenols, the anesthetic propofol (2,6-diisopropylphenol, P) and the anti-inflammatory diflunisal (2′,4′-difluoro-4-hydroxy-3-biphenyl carboxylic acid, D) also enhanced [3H]MUS binding, decreased the EC50 values for GABA in enhancing [3H]FNM binding and potentiated the enhancing effect of 200 nM GABA on [3H]FNM binding, although enhancements of [3H]MUS binding for these monophenols were smaller than those for H and M, using forebrain and cerebellar membranes. The enhancing effect of P and D on [3H]MUS binding were almost completely additive. 2,2′-biphenol was inactive on [3H]MUS and [3H]FNM binding. These, and other preliminary experiments, suggest that appropriate ortho (C2) and para (C4) substitution increases the GABA-potentiating activity of phenols. 6. The potentiation of GABAergic neurotransmission by H and M is probably involved in their previously reported anxiolytic and central depressant effects.
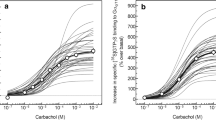
Similar content being viewed by others
REFERENCES
Chan, K. 1995. Progress in traditional Chinese medicine. Trends Pharmacol. Sci. 16:182–187.
Clark, A. M. 1996. Natural products as a resource for new drugs. Pharmaceut. Res. 13:1133–1141.
Ding, Y., and He, X. 1986. Traditional Chinese herbs in treatment of neurological and neurosurgical disorders. Can. J. Neural. Sci. 13:210–213.
Shen, M. L. 1983. The contribution of traditional Chinese medicine to modern pharmacology. Trends Pharmacol. Sci. 4:496–500.
Wang, Z. T., Ng, T. B., and Xu, G. J. 1995. Recent advances in pharmacognosy research in China. Gen. Pharmacol. 26:1221–1224.
Xiao, P. 1983. Recent developments on medicinal plants in China. J. Ethnopharm. 7:95–109.
Farnsworth, N. R. 1993. Ethnopharmacology and future drug development: The North American experience. J. Ethnopharmacol. 38:145–152.
Fujita, M., Itokawa, H., and Sashida, Y. 1972. Honokiol, a new phenolic compound isolated from the bark of Magnolia Obovata Thunb. Chem. Pharm. Bull. 20:212–213.
Sarker, S. D. 1997. Biological activity of magnolol: A review. Fitoterapia, LXVIII:3–8.
Watanabe, K., Watanabe, H., Goto, Y., and Yamaguchi, M. 1983. Pharmacological properties of magnolol and honokiol extracted from magnolia officinales: central depressant effects. Planta. Med. 49:103–108.
Maruyama, Y., Kuribara, H., Morita, M., Yuzurihara, M., and Weintraub, S. 1998. Identification of magnolol and honokiol as anxiolytic agents in extracts of Saiboku-to, an oriental herbal medicine. J. Nat. Prod. 61:135–138.
Tsai, T. H., Westly, J., Lee, T. F., Chen, C. F., and Wang, L. C. H. 1995. Effects of honokiol and magnolol on acetycholine release from rat hippocampal slices. Planta. Med. 61:477–479.
Tsai, T. H., Westly, J., Lee, T. F., Chen, C. F., and Wang, L. C. H. 1995. Modulatory effects of magnolol on potassium-stimulated 5-hydroxytryptamine release from rat cortical and hippocampal slices. Neurosci. Lett. 186:49–52.
Nielsen, M., Witt, M. R., and Thøgersen, H. 1988. [3H]Diazepam specific binding to rat cortex in vitro is enhanced by oleic, arachidonic and docosahexenoic acid isolated from pig brain. Eur. J. Pharmacol. 146:349–353.
Witt, M. R., Westh-Hansen, S. E., Rasmussen, P. B., Hastrup, S., and Nielsen, M. 1996. Unsaturated free fatty acids increase benzodiazepine receptor agonist binding depending on the subunit composition of the GABAA receptor complex. J. Neurochem. 67:2141–2145.
Ai, J., Dekermendjian, K., Wang, X., Nielsen, M., and Witt, M. R. 1997. 6-methylflavone, a benzodiazepine receptor ligand with antagonistic properties on rat brain and human recombinant GABAA receptors in vitro. Drug Dev. Res. 41:99–106.
Squires, R. F., and Saederup, E. 1982. γ-Aminobutyric acid receptors modulate cation binding sites coupled to independent benzodiazepine, picrotoxin, and anion binding sites. Mol. Pharmacol. 22:327–334.
Squires, R. F., Casida, J. E., Richardson, M., and Saederup, E. 1983. [35S]-t-Butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to γ-aminobutyric acid-A and ion recognition sites. Mol. Pharmacol. 23:326–336.
Squires, R. F., and Saederup, E. 1999. Clozapine's antipsychotic effects do not depend on blockade of 5-HT3 receptors. Neurochem. Res. 24:659–667.
Login, I. S., Pal, S. H., Adams, D. T., and Gold, P. E. 1998. Muscimol increases acetylcholine release by directly stimulating adult striatal cholinergic interneurons. Brain Res. 779:33–40.
Kuribara, H., Stavinoha, W. B., and Maruyama, Y. 1999. Honokiol, a putative anxiolytic agent extracted from magnolia bark, has no diazepam-like side-effects in mice. J. Pharm. Pharmacol. 51:97–103.
McKernan, R., and Whiting, P. J. 1996. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 19:139–143.
Sieghart, W. 1995. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol. Rev. 47:181–234.
Johnston, G. A. R. 1996. GABAA receptor pharmacology. Pharmacol. Ther. 69:173–198.
Rabow, L. E., Russek, S., and Farb, K. H. 1995. From ion currents to genomic analysis: Recent advances in GABAA receptor research. Synapse 21:189–274.
Kerr, D. I. B., and Ong, J. 1992. GABA agonists and antagonists. Med. Res. Rev. 12:593–636.
Ai, J., Dekermendjian, K., Nielsen, M., and Witt, M. R. 1997. The heteroyohimbine mayumbine binds with high affinity to rat brain benzodiazepine receptors in vitro. Nat. Prod. Lett. 11:73–76.
Ai, J., Dekermendjian, K., Sterner, O., Nielsen, M., and Witt, M. R. 1997. Compounds isolated from medicinal plants as ligands for benzodiazepine receptors. Recent Adv. Phytochem. 1:365–385.
Dekermendjian, K., Ai, J., Nielsen, M., Sterner, O., Shan, R., and Witt, M. R. 1996. Characterisation of furanocoumarin phellopterin as a rat brain benzodiazepine receptor partial agonist in vitro. Neurosci. Lett. 219:151–154.
Medina, J. H., Viola, H., Wolfman, C., Marder, M., Wasowske, C., Calvo, D., and Paladini, A. C. 1997. Overview-Flavonoids: A new family of benzodiazepine receptor ligands. Neurochem. Res. 22:419–425.
Ebert, B., Frølund, B., Diemer, N. H., and Krogsgaard-Larsen, P. 1999. Equilibrium binding characteristics of [3H]thiomuscimol. Neurochem. Int. 34:427–434.
Asano, T., and Ogasawara, N. 1981. Chloride-dependent stimulation of GABA and benzodiazepine receptor binding by pentobarbital. Brain Res. 225:212–216.
Olsen, R. W., and Snowman, A. M. 1982. Chloride-dependent enhancement by barbiturates of γ-aminobutyric acid receptor binding. J. Neurosci. 2:1812–1823.
Peters, J. A., Kirkness, E. F., Callachan, H., Lambert, J. J., and Turner, A. J. 1988. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Brit. J. Pharmacol. 94:1257–1269.
Jussofie, A., Schmiz, A., and Hiemke, C. 1994. Kavapyrone enriched extract from Piper methysticum as modulator of the GABA binding site in different regions of rat brain. Psychopharmacology 116:469–474.
Woodward, R. M., Polenzani, L., and Miledi, R. 1994. Effects of fenamates and other nonsteroidal anti-inflammatory drugs on rat brain GABAA receptors expressed in xenopus oocytes. J. Pharmacol. Exp. Ther. 268:806–817.
Hasan, Z. A., and Woolley, D. E. 1999. The short-acting anesthetic propofol produces biphasic effects — depression and withdrawal rebound overshoot — on some (but not all) limbic evoked potentials in the behaving rat. Brain Res. 818:51–64.
Trapani, G., Latrofa, A., Franco, M., Altomare, C., Sanna, E., Usala, M., Biggio, G., and Liso, G. 1998. Propofol analogues. Synthesis, relationships between structure and affinity at GABAA receptor in rat brain, and differential electrophysiological profile at recombinant human GABAA receptors. J. Med. Chem. 41:1846–1854.
Nielsen, N., Frøkjaer, S., and Braestrup, C. 1988. High affinity of the naturally-occurring biflavonoid, amentoflavon, to brain benzodiazepine receptors in vitro. Biochem. Pharmacol. 37:3285–3287.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Squires, R.F., Ai, J., Witt, MR. et al. Honokiol and Magnolol Increase the Number of [3H]Muscimol Binding Sites Three-Fold in Rat Forebrain Membranes In Vitro Using a Filtration Assay, by Allosterically Increasing the Affinities of Low-Affinity Sites. Neurochem Res 24, 1593–1602 (1999). https://doi.org/10.1023/A:1021116502548
Issue Date:
DOI: https://doi.org/10.1023/A:1021116502548