Abstract
Objective
To compare the effectiveness of intermittent with daily chemotherapy (both containing rifampicin) in childhood tuberculosis (age ≤16yrs) in achieving cure/significant improvement.
Design
Systematic Review and Meta-analysis.
Methods
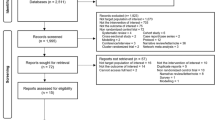
MEDLINE and the Cochrane Library were searched for randomized trials of antitubercular regimens containing rifampicin, in children 16 yrs or less with tuberculosis. Two reviewers independently assessed trial eligibility and quality. Data from full articles of selected studies were independently extracted by two authors and analyzed. The odds ratio was obtained for the pooled data in two groups (intermittent and daily therapy).
Outcome variables
Cure/significant improvement, relapse rate and adverse events.
Results
Four randomized controlled trials comparing twice weekly and daily therapy including 466 children pulmonary 439; extrapulmonary 27) met the inclusion criteria. Baseline data were comparable. On quality assessment, 3 studies scored 2 and one study scored 3 out of 5 points. Per protocol analysis showed that children receiving intermittent regimen were less likely to be cured than those receiving daily therapy (OR 0.27; 95% CI: 0.14, 0.51). The results of intention to treat analysis suggest similar trend towards lower cure rates with twice weekly regimen (OR 0.66; 95% CI: 0.23–1.84).
Conclusion
Twice weekly intermittent short course therapy is less likely to cure tuberculosis in children as compared to daily therapy. There is a need for better quality randomized controlled trials for assessing efficacy of alternate schedule for intermittent therapy for childhood tuberculosis.
Similar content being viewed by others
References
Cohn DL, Catlin BJ, Peterson KL, Judson FN, Sbarbaro JA. A 62-dose, 6-month therapy for pulmonary and extrapulmonary tuberculosis. A twice-weekly, directly observed and cost-effective regimen. Ann Intern Med 1990;112:407–415.
Caminero JA, Pavón JM, Rodríguez de Castro F, Díaz F, Julià G, Caylá JA, et al. Evaluation of a directly observed six month fully intermittent treatment regimens for tuberculosis in patients suspected of poor compliance. Thorax 1996;51:1130–1133.
Balasubramanian R. Fully intermittent six month regimens for pulmonary tuberculosis in south India. Indian J Tuberc 1991;38:51.
Bechan S, Connolly C, Short GM, Standing E, Wilkinson D. Directly observed therapy for tuberculosis given twice weekly in the workplace in urban South Africa. Trans Royal Soc Trop Med Hyg 1997;91:704–707.
CDC core curriculum: treatment of TB Disease. http://www.umdnj.edu/~ntbcweb/coretrea.htm. Accessed on 12 December, 2007.
Abernathy RS, Dutt AK, Stead WW, Moers DJ. Short-course chemotherapy for tuberculosis in children. Pediatrics 1983;72:801–806.
Iseman MD, Cohn DL, Sbarbaro JA. Directly observed treatment of tuberculosis — we can’t afford not to try it. N Engl J Med 1993;328:576–578.
Dickersin K, Scherer R, Lefebvre C. Systematic reviews: Identifying relevant studies for systematic reviews. BMJ 1994;309:1286–1291.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of Randomized clinical trials: is blinding necessary? Controlled Clin Trials 1996;17:1–12.
RevMan Analyses [Computer program]. In: Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2003.
Dingley HB. Short-term chemotherapy in tuberculosis in children. Indian J Tuberc 1982;29:48–54.
Jawahar MS, Rajaram K, Sivasubramanian S, Paramasivan CN, Chandrasekar K, Kamaludeen MN, et al. Treatment of lymph node tuberculosis—a randomized clinical trial of two 6-month regimens. Trop Med Int Health 2005;10:1090–1098.
Rajeswari R, Sivasubramanian S, Balambal R, Parthasarathy R, Ranjani R, Santha T, et al. A controlled clinical trial of short-course chemotherapy for tuberculoma of the brain. Tuber Lung Dis 1995;76:311–317.
Balasubramanian R, Nagarajan M, Balambal R, Tripathy SP, Sundararaman R, Venkatesan P, et al. Randomised controlled clinical trial of short course chemotherapy in abdominal tuberculosis: a five-year report. Int J Tuberc Lung Dis 1997;1:44–51.
Anonymous. Controlled clinical trial of oral short-course regimens in the treatment of sputum-positive pulmonary tuberculosis. Tuberculosis Research Centre. Int J Tuberc Lung Dis 1997;1:509–517.
Swaminathan S, Raghavan A, Duraipandian M, Kripasankar AS, Ramachandran P. Short course chemotherapy for pediatric respiratory tuberculosis: 5-year report. Int J Tuberc Lung Dis 2005;9:693–696.
Ramachandran P, Kripasankar AS, Duraipandian M. Short Course Chemotherapy for pulmonary tuberculosis in children. Indian J Tuberc 1998;45:83–87.
Kansoy S, Kurtap N, Akpit S, Aksoylar S, Yaprak I, Çaðlayan S. Superiority of intermittent-short course chemotherapy in childhood pulmonary tuberculosis. Turkish J Med Sci 1996;26:41–43.
Kumar L, Dhand R, Singhi PD, Rao KL, Katariya S. A randomised trial of fully intermittent and daily followed by intermittent short-course chemotherapy for childhood tuberculosis. Pediatr Infect Dis J 1990;9:802–806.
Te Water Naude JM, Donald PR, Hussey GD, Kibel MA, Louw A, Perkins DR, et al. Twiceweekly vs daily chemotherapy for childhood TB. Pediatr Infect Dis J 2000;19:405–410.
World Health Organization. Provisional guidelines for the diagnosis and classification of the EPI target diseases for primary health care, surveillance and special studies. EPI/GEN/83/4. Geneva: WHO; 1983.
Mwandumba HC, Squire SB. Fully intermittent dosing with drugs for treating tuberculosis in adults. Cochrane Database Syst Rev 2001;4:CD000970.
Cox HS, Morrow M, Deutschmann PW. Long term efficacy of DOTS regimens for tuberculosis: systematic review. BMJ 2008;336:484–487.
Walley JD, Khan MA, Newell JN, Khan MH. Effectiveness of the direct observation component of DOTS for tuberculosis: a randomized controlled trial in Pakistan. Lancet 2001;357:664–669.
Snider DE, Graczyk J, Bek E, Rogowski J. Supervised six-months treatment of newly diagnosed pulmonary tuberculosis using isoniazid, rifampin, and pyrazinamide with and without streptomycin. Am Rev Respir Dis 1984;130:1091–1094.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramesh Menon, P., Lodha, R., Sivanandan, S. et al. Intermittent or daily short course chemotherapy for tuberculosis in children: Meta-analysis of randomized controlled trials . Indian Pediatr 47, 67–73 (2010). https://doi.org/10.1007/s13312-010-0009-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-010-0009-2