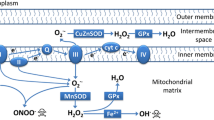
Abstract
Endothelial cells in vivo are constantly exposed to shear associated with blood flow and altered shear stress elicits cellular responses (mechanotransduction). This review describes the role of shear sensors and signal transducers in these events. The major focus is the response to removal of shear as occurs when blood flow is compromised (i.e., ischemia). Pulmonary ischemia studied with the isolated murine lung or flow adapted pulmonary microvascular endothelial cells in vitro results in endothelial generation of reactive oxygen species (ROS) and NO. The response requires caveolae and is initiated by endothelial cell depolarization via KATP channel closure followed by activation of NADPH oxidase (NOX2) and NO synthase (eNOS), signaling through MAP kinases, and endothelial cell proliferation. These physiological mediators can promote vasodilation and angiogenesis as compensation for decreased tissue perfusion.







Similar content being viewed by others
References
Szocs, K. (2004). Endothelial dysfunction and reactive oxygen species production in ischemia/reperfusion and nitrate tolerance. General Physiology and Biophysics, 23, 265–295.
Kutala, V. K., Khan, M., Angelos, M. G., & Kuppusamy, P. (2007). Role of oxygen in postischemic myocardial injury. Antioxidants & Redox Signaling, 9, 1193–1206.
McCord, J. M. (1985). Oxygen-derived free radicals in postischemic tissue injury. New England Journal of Medicine, 312, 159–163.
Spisni, E., Bianco, M. C., Griffoni, C., Toni, M., D’ Angelo, R., Santi, S., et al. (2003). Mechanosensing role of caveolae and caveolar constituents in human endothelial cells. Journal of Cellular Physiology, 197, 198–204.
Barakat, A. I., & Davies, P. F. (1998). Mechanisms of shear stress transmission and transduction in endothelial cells. Chest, 114, 58S–63S.
Lansman, J. B. (1988). Endothelial mechanosensors. Going with the flow. Nature, 331, 481–482.
Davies, P. F., & Tripathi, S. C. (1993). Mechanical stress mechanisms and the cell. An endothelial paradigm. Circulation Research, 72, 239–245.
Watson, P. A. (1991). Function follows form: Generation of intracellular signals by cell deformation. FASEB Journal, 5, 2013–2019.
Davies, P. F., Barbee, K. A., Volin, M. V., Robotewskyj, A., Chen, J., Joseph, L., et al. (1997). Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annual Review of Physiology, 59, 527–549.
Girard, P. R., & Nerem, R. M. (1993). Endothelial cell signaling and cytoskeletal changes in response to shear stress. Frontiers of Medical and Biological Engineering, 5, 31–36.
Wei, Z., Costa, K., Al-Mehdi, A. B., Dodia, C., Muzykantov, V., & Fisher, A. B. (1999). Simulated ischemia in flow-adapted endothelial cells leads to generation of reactive oxygen species and cell signaling. Circulation Research, 85, 682–689.
Chatterjee, S., Al-Mehdi, A. B., Levitan, I., Stevens, T., & Fisher, A. B. (2003). Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol, 285, C959–C967.
Kang-Decker, N., Cao, S., Chatterjee, S., Yao, J., Egan, L. J., Semela, D., et al. (2007). Nitric oxide promotes endothelial cell survival signaling through s-nitrosylation and activation of dynamin-2. Journal of Cell Science, 120, 492–501.
Fisher, A. B., Dodia, C., Tan, Z. T., Ayene, I., & Eckenhoff, R. G. (1991). Oxygen-dependent lipid peroxidation during lung ischemia. Journal of Clinical Investigation, 88, 674–679.
Ayene, I. S., Dodia, C., & Fisher, A. B. (1992). Role of oxygen in oxidation of lipid and protein during ischemia/reperfusion in isolated perfused rat lung. Archives of Biochemistry and Biophysics, 296, 183–189.
Al-Mehdi, A. B., Zhao, G., Dodia, C., Tozawa, K., Costa, K., Muzykantov, V., et al. (1998). Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+. Circulation Research, 83, 730–737.
Olesen, S. P., Clapham, D. E., & Davies, P. F. (1988). Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature, 331, 168–170.
Diamond, S. L., Sharefkin, J. B., Dieffenbach, C., Frasier-Scott, K., McIntire, L. V., & Eskin, S. G. (1990). Tissue plasminogen activator messenger rna levels increase in cultured human endothelial cells exposed to laminar shear stress. Journal of Cellular Physiology, 143, 364–371.
Davies, P. F. (1995). Flow-mediated endothelial mechanotransduction. Physiological Reviews, 75, 519–560.
Resnick, N., Collins, T., Atkinson, W., Bonthron, D. T., Dewey, C. F., Jr, & Gimbron M. A., Jr. (1993). Platelet-derived growth factor b chain promoter contains a cis-acting fluid shear-stress-responsive element. [Erratum for Proc Natl Acad Sci U.S.A. 1993 May 15;90(10):4591–4595; pmid: 8506304]. Proceedings of the National Academy of Sciences of the United States of America, 90, 7908.
Chien, S., Li, S., & Shyy, Y. J. (1998). Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension, 31, 162–169.
Davies, P. F. (2008). Endothelial transcriptome profiles in vivo in complex arterial flow fields. Annals of Biomedical Engineering, 36, 563–570.
Chien, S. (2006). Molecular basis of rheological modulation of endothelial functions: Importance of stress direction. Biorheology, 43, 95–116.
Labrador, V., Chen, K. D., Li, Y. S., Muller, S., Stoltz, J. F., & Chien, S. (2003). Interactions of mechanotransduction pathways. Biorheology, 40, 47–52.
Chen, K. D., Li, Y. S., Kim, M., Li, S., Yuan, S., Chien, S., et al. (1999). Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and shc. Journal of Biological Chemistry, 274, 18393–18400.
Liu, Y., Chen, B. P., Lu, M., Zhu, Y., Stemerman, M. B., Chien, S., et al. (2002). Shear stress activation of srebp1 in endothelial cells is mediated by integrins. Arteriosclerosis, Thrombosis, and Vascular Biology, 22, 76–81.
Wang, Y., Miao, H., Li, S., Chen, K. D., Li, Y. S., Yuan, S., et al. (2002). Interplay between integrins and flk-1 in shear stress-induced signaling. American Journal of Physiology—Cell Physiology, 283, C1540–C1547.
Kuchan, M. J., Jo, H., & Frangos, J. A. (1994). Role of g proteins in shear stress-mediated nitric oxide production by endothelial cells. American Journal of Physiology, 267, C753–C758.
Lieu, D. K., Pappone, P. A., & Barakat, A. I. (2004). Differential membrane potential and ion current responses to different types of shear stress in vascular endothelial cells. American Journal of Physiology—Cell Physiology, 286, C1367–C1375.
Traub, O., Ishida, T., Ishida, M., Tupper, J. C., & Berk, B. C. (1999). Shear stress-mediated extracellular signal-regulated kinase activation is regulated by sodium in endothelial cells. Potential role for a voltage-dependent sodium channel. Journal of Biological Chemistry, 274, 20144–20150.
Osawa, M., Masuda, M., Kusano, K., & Fujiwara, K. (2002). Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: Is it a mechanoresponsive molecule? Journal of Cell Biology, 158, 773–785.
Weinbaum, S., Zhang, X., Han, Y., Vink, H., & Cowin, S. C. (2003). Mechanotransduction and flow across the endothelial glycocalyx. Proceedings of the National Academy of Sciences of the United States of America, 100, 7988–7995.
Tzima, E., Irani-Tehrani, M., Kiosses, W. B., Dejana, E., Schultz, D. A., Engelhardt, B., et al. (2005). A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature, 437, 426–431.
Cooke, J. P., Rossitch, E., Jr, Andon, N. A., Loscalzo, J., & Dzau, V. J. (1991). Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. Journal of Clinical Investigation, 88, 1663–1671.
Hoger, U., & Seyfarth, E. A. (2001). Structural correlates of mechanosensory transduction and adaptation in identified neurons of spider slit sensilla. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 187, 727–736.
Chatterjee, S., Levitan, I., Wei, Z., & Fisher, A. B. (2006). KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation, 13, 633–644.
Milovanova, T., Chatterjee, S., Manevich, Y., Kotelnikova, I., Debolt, K., Madesh, M., et al. (2006). Lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species. American Journal of Physiology. Cell Physiology, 290, C66–C76.
Zhang, Q., Matsuzaki, I., Chatterjee, S., & Fisher, A. B. (2005). Activation of endothelial NADPH oxidase during normoxic lung ischemia is KATP channel dependent. Am J Physiol Lung Cell Mol Physiol, 289, L954–L961.
Milovanova, T., Chatterjee, S., Hawkins, B. J., Hong, N. K., Sorokina, E. M., DeBolt, K., Moore, J. S., Madesh, M., & Fisher, A. B. (2008). Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochimica et Biophysica Acta, 1783, 1866–1875.
Sukharev, S., & Corey, D. P. (2004). Mechanosensitive channels: Multiplicity of families and gating paradigms. Science’s Stke [Electronic Resource]: Signal Transduction Knowledge Environment; re4.
Maroto, R., Raso, A., Wood, T. G., Kurosky, A., Martinac, B., & Hamill, O. P. (2005). Trpc1 forms the stretch-activated cation channel in vertebrate cells [see comment]. Nature Cell Biology, 7, 179–185.
Geiger, B., Bershadsky, A., Pankov, R., & Yamada, K. M. (2001). Transmembrane crosstalk between the extracellular matrix—cytoskeleton crosstalk. Nature Reviews Molecular Cell Biology, 2, 793–805.
de Curtis, I., & Gatti, G. (1994). Identification of a large complex containing the integrin alpha 6 beta 1 laminin receptor in neural retinal cells. Journal of Cell Science, 107, 3165–3172.
Jalali, S., del Pozo, M. A., Chen, K., Miao, H., Li, Y., Schwartz, M. A., et al. (2001). Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ecm) ligands. Proceedings of the National Academy of Sciences of the United States of America, 98, 1042–1046.
Li, S., Kim, M., Hu, Y. L., Jalali, S., Schlaepfer, D. D., Hunter, T., et al. (1997). Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. Journal of Biological Chemistry, 272, 30455–30462.
Bhullar, I. S., Li, Y. S., Miao, H., Zandi, E., Kim, M., Shyy, J. Y., et al. (1998). Fluid shear stress activation of ikappab kinase is integrin-dependent. Journal of Biological Chemistry, 273, 30544–30549.
Radel, C., Carlile-Klusacek, M., & Rizzo, V. (2007). Participation of caveolae in beta1 integrin-mediated mechanotransduction. Biochemical and Biophysical Research Communications, 358, 626–631.
Kaufman, D. A., Albelda, S. M., Sun, J., & Davies, P. F. (2004). Role of lateral cell–cell border location and extracellular/transmembrane domains in pecam/cd31 mechanosensation. Biochemical and Biophysical Research Communications, 320, 1076–1081.
Manevich, Y., Al-Mehdi, A., Muzykantov, V., & Fisher, A. B. (2001). Oxidative burst and no generation as initial response to ischemia in flow-adapted endothelial cells. Am J Physiol Heart Circ Physiol, 280, H2126–H2135.
Al-Mehdi, A. B., Shuman, H., & Fisher, A. B. (1997). Intracellular generation of reactive oxygen species during nonhypoxic lung ischemia. American Journal of Physiology, 272, L294–L300.
Song, C., Al-Mehdi, A. B., & Fisher, A. B. (2001). An immediate endothelial cell signaling response to lung ischemia. American Journal of Physiology. Lung Cellular and Molecular Physiology, 281, L993–L1000, Corrigenda, 1282: Preceding L1167, 2002.
Al-Mehdi, A. B., Shuman, H., Fisher, A. B., Shuman, H., & Fisher, A. B. (1997). Oxidant generation with K(+)-induced depolarization in the isolated perfused lung. Free Radical Biology and Medicine, 23, 47–56.
Zhang, Q., Chatterjee, S., Wei, Z., Liu, W. D., & Fisher, A. B. (2008). Rac and pi3 kinase mediate endothelial cell-reactive oxygen species generation during normoxic lung ischemia. Antioxidants & Redox Signaling, 10, 679–689.
Tozawa, K., Al-Mehdi, A. B., Muzykantov, V., & Fisher, A. B. (1999). In situ imaging of intracellular calcium with ischemia in lung subpleural microvascular endothelial cells. Antioxidants Redox Signaling, 1, 145–154.
Matot, I., Manevich, Y., Al-Mehdi, A. B., Song, C., & Fisher, A. B. (2003). Fluorescence imaging of lipid peroxidation in isolated rat lungs during nonhypoxic lung ischemia. Free Radical Biology and Medicine, 34, 785–790.
Milovanova, T., Manevich, Y., Haddad, A., Chatterjee, S., Moore, J. S., & Fisher, A. B. (2004). Endothelial cell proliferation associated with abrupt reduction in shear stress is dependent on reactive oxygen species. Antioxidants Redox Signaling, 6, 245–258.
Matsuzaki, I., Chatterjee, S., Debolt, K., Manevich, Y., Zhang, Q., & Fisher, A. B. (2005). Membrane depolarization and NADPH oxidase activation in aortic endothelium during ischemia reflect altered mechanotransduction. American Journal of Physiology. Heart and Circulatory Physiology, 288, H336–H343.
Levitan, I., Helmke, B. P., & Davies, P. F. (2000). A chamber to permit invasive manipulation of adherent cells in laminar flow with minimal disturbance of the flow field. Annals of Biomedical Engineering, 28, 1184–1193.
Al-Mehdi, A. B., Zhao, G., & Fisher, A. B. (1998). ATP-independent membrane depolarization with ischemia in the oxygen-ventilated isolated rat lung. American Journal of Respiratory Cell and Molecular Biology, 18, 653–661.
Al-Mehdi, A. B., Zhao, G., Tozawa, K., & Fisher, A. B. (2000). Depolarization-associated iron release with abrupt reduction in pulmonary endothelial shear stress in situ. Antioxidants Redox Signaling, 2, 335–345.
Wei, Z., Al-Mehdi, A. B., & Fisher, A. B. (2001). Signaling pathway for nitric oxide generation with simulated ischemia in flow-adapted endothelial cells. American Journal of Physiology. Heart and Circulatory Physiology, 281, H2226–H2232.
Wei, Z., Manevich, Y., Al-Mehdi, A. B., Chatterjee, S., & Fisher, A. B. (2004). Ca2+ flux through voltage-gated channels with flow cessation in pulmonary microvascular endothelial cells. Microcirculation, 11, 517–526.
Haugland, R. P. (1996). Handbook of fluorescent probes and research chemicals (6th ed.). Eugene, OR.
Al-Mehdi, A. B., Ischiropoulos, H., & Fisher, A. B. (1996). Endothelial cell oxidant generation during K(+)-induced membrane depolarization. Journal of Cellular Physiology, 166, 274–280.
Al-Mehdi, A. B., Song, C., Tozawa, K., & Fisher, A. B. (2000). Ca2+-and phosphatidylinositol 3-kinase-dependent nitric oxide generation in lung endothelial cells in situ with ischemia. Journal of Biological Chemistry, 275, 39807–39810.
Zhao, G., Al-Mehdi, A. B., & Fisher, A. B. (1997). Anoxia-reoxygenation versus ischemia in isolated rat lungs. American Journal of Physiology, 273, L1112–L1117.
Jones, S. A., O’Donnell, V. B., Wood, J. D., Broughton, J. P., Hughes, E. J., & Jones, O. T. (1996). Expression of phagocyte NADPH oxidase components in human endothelial cells. American Journal of Physiology, 271, H1626–H1634.
Babior, B. M. (2000). The NADPH oxidase of endothelial cells. IUBMB Life, 50, 267–269.
Zulueta, J. J., Sawhney, R., Yu, F. S., Cote, C. C., & Hassoun, P. M. (1997). Intracellular generation of reactive oxygen species in endothelial cells exposed to anoxia-reoxygenation. American Journal of Physiology, 272, L897–L902.
Wu, S., Haynes, J., Jr, Taylor, J. T., Obiako, B. O., Stubbs, J. R., Li, M., et al. (2003). Cav3.1 (alpha1 g) t-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circulation Research, 93, 346–353.
Rizzo, V., Sung, A., Oh, P., & Schnitzer, J. E. (1998). Rapid mechanotransduction in situ at the luminal cell surface of vascular endothelium and its caveolae. Journal of Biological Chemistry, 273, 26323–26329.
Rizzo, V., Morton, C., DePaola, N., Schnitzer, J. E., & Davies, P. F. (2003). Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. American Journal of Physiology. Heart and Circulatory Physiology, 285, H1720–H1729.
Yu, J., Bergaya, S., Murata, T., Alp, I. F., Bauer, M. P., Lin, M. I., et al. (2006). Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels [see comment]. Journal of Clinical Investigation, 116, 1284–1291.
Sedding, D. G., Hermsen, J., Seay, U., Eickelberg, O., Kummer, W., Schwencke, C., et al. (2005). Caveolin-1 facilitates mechanosensitive protein kinase b (Akt) signaling in vitro and in vivo. Circulation Research, 96, 635–642.
Oh, P., & Schnitzer, J. E. (2001). Segregation of heterotrimeric g proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas g(i) and g(s) target lipid rafts by default. Molecular Biology of the Cell, 12, 685–698.
Zhu, Y., Liao, H. L., Wang, N., Yuan, Y., Ma, K. S., Verna, L., et al. (2000). Lipoprotein promotes caveolin-1 and ras translocation to caveolae: Role of cholesterol in endothelial signaling. Arteriosclerosis, Thrombosis, and Vascular Biology, 20, 2465–2470.
Gorodinsky, A., & Harris, D. A. (1995). Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. Journal of Cell Biology, 129, 619–627.
Grill, H. P., Zweier, J. L., Kuppusamy, P., Weisfeldt, M. L., & Flaherty, J. T. (1992). Direct measurement of myocardial free radical generation in an in vivo model: Effects of postischemic reperfusion and treatment with human recombinant superoxide dismutase. Journal of the American College of Cardiology, 20, 1604–1611.
Udassin, R., Ariel, I., Haskel, Y., Kitrossky, N., & Chevion, M. (1991). Salicylate as an in vivo free radical trap: Studies on ischemic insult to the rat intestine. Free Radical Biology and Medicine, 10, 1–6.
Sohn, H. Y., Keller, M., Gloe, T., Morawietz, H., Rueckschloss, U., & Pohl, U. (2000). The small g-protein rac mediates depolarization-induced superoxide formation in human endothelial cells. Journal of Biological Chemistry, 275, 18745–18750.
Inoue, J., Gohda, J., Akiyama, T., & Semba, K. (2007). Nf-kappa b activation in development and progression of cancer. Cancer Science, 98, 268–274.
Sunters, A., Fernandez de Mattos, S., Stahl, M., Brosens, J. J., Zoumpoulidou, G., Saunders, C. A., et al. (2003). Foxo3a transcriptional regulation of bim controls apoptosis in paclitaxel-treated breast cancer cell lines. Journal of Biological Chemistry, 278, 49795–49805.
Meloche, S., & Pouyssegur, J. (2007). The erk1/2 mitogen-activated protein kinase pathway as a master regulator of the g1- to s-phase transition. Oncogene, 26, 3227–3239.
Wagner, E. M., Petrache, I., Schofield, B., & Mitzner, W. (2006). Pulmonary ischemia induces lung remodeling and angiogenesis. Journal of Applied Physiology, 100, 587–593.
Ushio-Fukai, M. (2007). VEGF signaling through NADPH oxidase-derived ROS. Antioxidants & Redox Signaling, 9, 731–739.
Cameron, A. R., Nelson, J., & Forman, H. J. (1983). Depolarization and increased conductance precede superoxide release by concanavalin a-stimulated rat alveolar macrophages. Proceedings of the National Academy of Sciences of the United States of America, 80, 3726–3728.
Korchak, H. M., & Weissmann, G. (1978). Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proceedings of the National Academy of Sciences of the United States of America, 75, 3818–3822.
Acknowledgments
We thank Susan Turbitt for secretarial support and the many collaborators who have contributed to this research during the past 20 years. Original research has been supported by the NHLBI (HL79063, HL60290, and HL41939).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chatterjee, S., Chapman, K.E. & Fisher, A.B. Lung Ischemia: A Model for Endothelial Mechanotransduction. Cell Biochem Biophys 52, 125–138 (2008). https://doi.org/10.1007/s12013-008-9030-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-008-9030-7