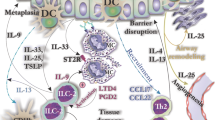
Abstract
Interleukin-13 is a pleiotropic TH2 cytokine that has been shown to be central to the pathogenesis of asthma. Some of the most prominent of the effects of IL-13 include increases in goblet cell differentiation, activation of fibroblasts, elevation of bronchial hyperresponsiveness, and switching of B cell antibody production from IgM to IgE. The relevances of these effects to asthma have been carefully studied in both animal models and more recently in human studies. As the role of IL-13 in asthma has become more defined, a number of potential biomarkers for TH2 airway inflammation, and hence IL-13 activity, have been identified, including blood and sputum eosinophils, total serum IgE, proteins derived from the bronchial epithelium (e.g., serum periostin), and exhaled nitric oxide. Most importantly, many of these markers for TH2 inflammation are strong predictors for positive responses to inhaled corticosteroid treatment. These biomarkers may also be useful in identifying patients who are most likely to benefit from specific IL-13 antagonism, as was demonstrated in a recent clinical trial of anti-IL-13 antibody therapy (lebrikizumab) in patients with poorly controlled asthma despite using inhaled corticosteroids. In that study, significant improvements in FEV1 were observed in patients with elevations of serum periostin but not in patients with normal periostin levels. These data indicate that IL-13 antagonists may fulfill an important unmet need in patients with poorly controlled asthma and biologic evidence of persistent IL-13 activity.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Pushparaj PN, Tay HK, H’ng SC, et al. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A. 2009;106(24):9773–8.
Hurst SD, Muchamuel T, Gorman DM, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169(1):443–53.
Krause S, Behrends J, Borowski A, et al. Blockade of interleukin-13-mediated cell activation by a novel inhibitory antibody to human IL-13 receptor alpha1. Mol Immunol. 2006;43(11):1799–807.
Punnonen J, Aversa G, Cocks BG, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–4.
Horie S, Okubo Y, Hossain M, et al. Interleukin-13 but not interleukin-4 prolongs eosinophil survival and induces eosinophil chemotaxis. Intern Med. 1997;36:179–85.
Luttmann W, Knoechel B, Foerster M, et al. Activation of human eosinophils by IL-13. Induction of CD69 surface antigen, its relationship to messenger RNA expression, and promotion of cellular viability. J Immunol. 1996;157:1678–83.
Ramirez-Icaza G, Mohammed KA, Nasreen N. Th2 cytokines IL-4 and IL-13 downregulate paxillin expression in bronchial airway epithelial cells. J Clin Immunol. 2004;24(4):426–34.
Kondo M, Tamaoki J, Takeyama K, et al. Elimination of IL-13 reverses established goblet cell metaplasia into ciliated epithelia in airway epithelial cell culture. Allergol Int. 2006;55:329–36.
Chibana K, Trudeau JB, Mustovich AT, et al. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy. 2008;8:936–46.
Richter A, Puddicombe SM, Lordan JL, et al. The contribution of interleukin (IL)-4 and IL-13 to the epithelial–mesenchymal trophic unit in asthma. Am J Respir Cell Mol Biol. 2001;25:385–91.
Bossé Y, Thompson C, Audette K, Stankova J, Rola-Pleszczynski M. Interleukin-4 and interleukin-13 enhance human bronchial smooth muscle cell proliferation. Int Arch Allergy Immunol. 2008;146(2):138–48.
Chiba Y, Nakazawa S, Todoroki M, Shinozaki K, Sakai H, Misawa M. Interleukin-13 augments bronchial smooth muscle contractility with an up-regulation of RhoA protein. Am J Respir Cell Mol Biol. 2009;40(2):159–67.
Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283(5405):1183–6.
Skowron-zwarg M, Boland S, Caruso N, Coraux C, Marano F, Tournier F. Interleukin-13 interferes with CFTR and AQP5 expression and localization during human airway epithelial cell differentiation. Exp Cell Res. 2007;313:2695–702.
Chiba Y, Goto K, Misawa M. Interleukin-13-induced activation of signal transducer and activator of transcription 6 is mediated by an activation of Janus kinase 1 in cultured human bronchial smooth muscle cells. Pharmacol Rep. 2012;64(2):454–8.
Zheng T, Liu W, Oh SY, et al. IL-13 receptor alpha2 selectively inhibits IL-13-induced responses in the murine lung. J Immunol. 2008;180:522–9.
Grunig G, Warnock M, Wakil AER, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3.
Prieto J, Lensmar C, Roquet AI, et al. Increased interleukin-13 mRNA expression in bronchoalveolar lavage cells of atopic patients with mild asthma after repeated low-dose allergen provocations. Respir Med. 2000;94:806–14.
Howard TD, Koppelman GH, Xu J, et al. Gene-gene interaction in asthma: IL4RA and IL13 in a Dutch population with asthma. Am J Hum Genet. 2002;70:230–6.
Li X, Howard TD, Zheng SL, et al. Genome-wide association study of asthma identifies RAD50-IL13 and HLA-DR/DQ regions. J Allergy Clin Immunol. 2010;125:328–35.
Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61.
Berry M, Morgan A, Shaw DE, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–9.
Woodruff PG, Modrek B, Choy DF, Jia G, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95.
•• Jia G, Erickson RW, Choy DF, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. Bronchoscopic Exploratory Research Study of Biomarkers in Corticosteroid-refractory Asthma (BOBCAT) Study Group. J Allergy Clin Immunol. 2012;130(3):647–54. Paper presents experimental evidence that periostin is linked to IL-13 and TH2 phenotype.
Woodruff PG, Khashayar R, Lazarus SC, et al. Relationship between airway inflammation, hyperresponsiveness, and obstruction in asthma. J Allergy Clin Immunol. 2001;108(5):753–8.
Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160(3):1001–8.
Spanevello A, Confalonieri M, Sulotto F, et al. Induced sputum cellularity. Reference values and distribution in normal volunteers. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1172–4.
McGrath KW, Icitovic N, Boushey HA, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185(6):612–9.
Saha SK, Berry MA, Parker D, et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121(3):685–91.
Kuiper S, Muris JW, Dompeling E, et al. Association between first-degree familial predisposition of asthma and atopy (total IgE) in newborns. Clin Exp Allergy. 2006;36(5):594–601.
Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104.
Warke TJ, Fitch PS, Brown V, et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002;57(5):383–7.
Payne DN, Adcock IM, Wilson NM, et al. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1376–81.
Berry MA, Shaw DE, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy. 2005;35(9):1175–9.
van den Toorn LM, Overbeek SE, de Jongste JC, Leman K, Hoogsteden HC, Prins JB. Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med. 2001;164(11):2107–13.
Smith AD, Cowan JO, Brassett KP, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med. 2005;172(4):453–9.
Cowan DC, Hewitt RS, Cowan JO, et al. Exercise-induced wheeze: fraction of exhaled nitric oxide-directed management. Respirology. 2010;15(4):683–90.
Zacharasiewicz A, Wilson N, Lex C, et al. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med. 2005;171(10):1077–82.
Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–84.
•• Corren J, Lemanske RF, Hanania N, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–98. First paper to demonstrate that IL-13 inhibition has clinical benefits in asthma, and that this improvement was largely determined by the presence of periostin.
Piper E, Brightling C, Niven R, et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J. 2013;41(2):330–8.
Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370(9596):1422–31.
Slager RE, Otulana BA, Hawkins GA, et al. IL-4 receptor polymorphisms predict reduction in asthma exacerbations during response to an anti-IL-4 receptor α antagonist. J Allergy Clin Immunol. 2012;130(2):516–22.
Corren J, Busse W, Meltzer EO, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181(8):788–96.
Gauvreau GM, Boulet LP, Cockcroft DW, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med. 2011;183:1007–14.
Kasaian MT, Raible, Marquette DK, et al. IL-13 antibodies influence IL-13 clearance in humans by modulating scavenger activity of IL-13Ralpha2. J Immunol. 2011;187:561–9.
Hodsman P, Ashman C, Cahn A, et al. A phase 1, randomized, placebo-controlled, dose-escalation study of an anti-IL-13 monoclonal antibody in healthy subjects and mild asthmatics. Br J Clin Pharmacol. 2013;75(1):118–28.
Compliance with Ethics Guidelines
Conflict of Interest
Jonathan Corren declares that he has the following conflicts of interest: Consultant, speaker, and recipient of research funding form Genentech. Consultant and recipient of research funding from Sanofi, Regeneron, and Medimmune.
Human and Animal Rights and Informed Consent
This article does not contain any studies with animal subjects performed by the author. With regard to the author’s research cited in this paper, all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corren, J. Role of Interleukin-13 in Asthma. Curr Allergy Asthma Rep 13, 415–420 (2013). https://doi.org/10.1007/s11882-013-0373-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11882-013-0373-9