Abstract
Purpose
To describe the practice, knowledge and beliefs about aerosol therapy during mechanical ventilation in an international sample of physicians working in intensive care units (ICU).
Methods
A self-administered survey was emailed to physicians who worked regularly in ICUs. The physicians were identified from the databases of the European and French societies of intensive care medicine and the REVA network.
Results
Of the 1,192 responses (15 % response rate), 854 were analyzed. Of the respondents, who represented 611 departments in 70 countries, 99 % reported using aerosol therapy during mechanical ventilation (including non-invasive), 43 % exclusively used nebulizers and 55 % also used metered dose inhalers. Nebulization relied on jet, ultrasonic and vibrating mesh nebulizers (55 %, 44 % and 14 % of respondents, respectively). Bronchodilators and steroids were the most frequently delivered drugs, and 80 % of respondents had a positive opinion concerning nebulized colistin and 30 % reported the use of nebulized antibiotics at least every other month. During nebulization, ventilator settings were never changed by 77 % of respondents, 65 % reported placing a filter on the expiratory limb, and of these 28 % never changed it. Only 22 % of respondents using heated humidifiers reported turning them off during nebulization. Specific knowledge about droplet size and nebulization yield was poor. A majority of respondents (87 %) thought that ultrasonic nebulizers outperform jet nebulizers, while 69 % had no opinion concerning mesh nebulizers.
Conclusions
Aerosol therapy during mechanical ventilation is used by over 95 % of intensivists, mostly for bronchodilator and steroid administration, but also frequently for antibiotics. The current scientific knowledge about optimal implementation seemed infrequently applied, suggesting the need for educational programs and research focusing on a better bench-to-bedside transfer of knowledge.
Similar content being viewed by others
Introduction
Administration of aerosolized medication to treat various pulmonary diseases is common practice. In particular, inhaled bronchodilators and steroids are part of the recommended maintenance therapy for patients with obstructive pulmonary disease as this route of delivery aims at reaching high drug concentrations at the site of action while limiting systemic exposure [1]. More recently, inhaled antibiotics have been successfully used to treat tracheobronchial infections in outpatients with stable cystic fibrosis [2]. In critically ill patients undergoing mechanical ventilation (MV), the use of inhaled drugs, albeit appealing, has been hampered by the low amount of drug available to the patient after inhalation. Indeed, the endotracheal tube and ventilator circuit were found to trap most of the aerosolized drug, allowing less than 5–10 % to reach the patient [3–5]. Subsequently, great research effort was focused on understanding the factors governing aerosol delivery during MV which are now relatively well described (ventilator setting, circuit setup and humidification) [6–11].
The implementation of aerosol therapy during MV was also simplified through technological advances such as the development of ultrasonic and vibrating mesh nebulizers [10]. Indeed, nowadays, most modern ventilators offer an integrated jet, ultrasonic or vibrating mesh nebulization system. The use of metered dose inhalers was also simplified through the development of inhalation chambers with inhaler access ports that are simple to adapt to ventilator circuits [12]. These advances led to an improvement in the performance of aerosol therapy during MV, generating new interest and potential new applications such as inhaled antibiotic treatment for ventilator-associated pneumonia [13–17]. Thus, nowadays, aerosol therapy to treat various pulmonary diseases during MV may be appropriate, but implementation may be relatively complex due to multiple possible combinations of aerosolization device, ventilator settings, molecules and indications [18]. Further, the widespread use of non-invasive ventilation constitutes a new challenge for aerosol therapy [19].
The current practice of aerosol therapy during invasive and non-invasive MV is unknown. In the outpatient setting, despite numerous guidelines, educational programs and widespread use, knowledge about aerosol therapy among physicians remains poor [20]. Knowledge among intensive care unit physicians dealing with the increased complexity of critically ill patients and MV has not yet been evaluated. The aim of this electronic survey was to determine the current practice, knowledge and opinions of physicians working in intensive care medicine about aerosol therapy during MV.
Materials and methods
This physician self-administered email-based cross-sectional survey was carried out from June to September 2011. The survey was endorsed by the European Critical Care Research Network (ECCRN) of the European Society of Intensive Care Medicine (ESICM) and the REVA network (Réseau Européen de recherche en Ventilation Artificielle). The questionnaire, comprising 38 questions, was developed through question-item generation/reduction performed to fit the survey objectives and practicability [21]. Items were organized within five domains: (1) aerosolization devices (jet, ultrasonic, vibrating mesh nebulizers, and metered dose inhalers); (2) drugs (bronchodilators, steroids, antibiotics); (3) ventilator and circuit (settings, device placement, humidification); (4) non-invasive ventilation; and (5) knowledge and beliefs. Actual knowledge and/or recommendations on this topic were also reviewed and are summarized in Fig. 1 (for example: jet, ultrasonic and vibrating mesh nebulizers are all effective in delivering bronchodilators to the airways; when targeting the alveolar region for delivering antibiotics it may be necessary to optimize nebulization settings to favor delivery of small droplets (1–3 μm) and avoid medication loss in the ventilator circuit by ventilator setting adjustments, humidification interruption, or optimal placement in the circuit).
Analysis was performed using descriptive statistics and data are reported as medians (25th to 75th percentiles), counts and percentages (see Electronic Supplementary Material for a more detailed discussion of the methods).
Results
Study respondents
Responses were received from 1,192 physicians (15 % response rate). The analysis included the responses from 854 physicians who completed more than 70 % of the questionnaire and regularly worked in an intensive care or intermediate care unit. This sample represented 611 departments in 70 countries (France 42 % and rest of Europe 36 %, and of the remaining (22 %), 16 % were from north America, 24 % from south America, 40 % from Asia, 9 % from Africa and 11 % from Australia/New Zealand). A majority of respondents (n = 745, 87 %) were board-certified physicians, working in an adult setting (n = 48 paediatricians or neonatologists) of mixed (62 %) or medical intensive care (21 %).
Aerosolization devices
Of the 854 respondents, 6 (<1 %) reported never using aerosol therapy during MV, 18 (2 %) exclusively used metered dose inhalers, 367 (43 %) exclusively nebulizers and 463 (54 %) both types of devices. Nebulization was mostly performed using a jet nebulizer (456 respondents, 55 %) or ultrasonic nebulizer (365 respondents, 44 %), and less frequently using a vibrating mesh nebulizer (116 respondents, 14 %; the total exceeds 100 % due to the use of multiple devices). The distribution was similar when analyzing departments rather than respondents (data not shown).
For jet nebulization, 55 respondents (14 %) reported using an external gas source at least sometimes, whereas 252 respondents (65 %) would always use ventilator-integrated systems, if available. In the absence of a ventilator-integrated jet nebulization system, 154 respondents (40 %) reported using an external gas source, the remainder changing the ventilator (17 %) or using another aerosolization technique (43 %).
Metered dose inhalers were used by 481 respondents (56 %) during MV, directly into the tracheal tube after disconnecting the patient (80 respondents, 17 %) or via an inhalation chamber (365 respondents, 78 %) placed within the circuit either before (43 %) or after (57 %) the Y piece.
Drugs
Bronchodilators and steroids were the most frequently aerosolized drugs during MV (Table 1; Fig. 2). About 30 % of respondents reported nebulization of antibiotics in more than five patients a year (approximately one every other month) and, in some departments (85, 14 %), this was a frequent practice (several patients a month), in particular for colistin (Fig. 2).
Drugs delivered as an aerosol during invasive MV. Data are presented as percentage of respondents (n = 816) reporting delivering each drug as an aerosol during invasive MV either never, exceptionally (<5 patients/year), usually (5–12 patients/year) or frequently (>1 patient/month). For each drug the absolute number of respondents reporting at least exceptional use is indicated on the left
Ventilator settings and circuit
Most of the respondents (632, 77 %) reported never changing ventilator settings because of nebulization, whereas 187 (23 %) would always try to change them. Among the latter, 30 (16 %) reported doing so only in deeply sedated and/or paralyzed patients, 71 (38 %) sometimes administering sedatives or muscle relaxants in order to change the settings, whereas 79 (42 %) reported not changing them in pressure support. An increase in inspiratory time was the most frequently reported change (always or frequently done by 80 respondents, 48 %). The use of sedatives was not evaluated separately from ventilator settings (sedation as a means of improving aerosol delivery through improved patient–ventilator synchrony).
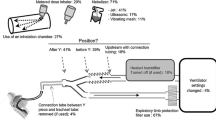
Placement of nebulizers, connection tubing and filters are illustrated in Fig. 3 and detailed in the Electronic Supplementary Material.
Ventilator circuit setup. The values indicate the percentage of respondents (n = 820). Most of the respondents reported placing the nebulizer just before or just after the Y piece, 57 % leaving the connection tubing between the Y piece and the tracheal tube in place during nebulization. Among 65 % of respondents reporting placing a filter on the expiratory limb, 28 % reported never changing it (see Electronic Supplementary Material)
Among units using heated humidifiers, only 22 % of respondents (n = 136) reported stopping heated humidification systems during nebulization.
Non-invasive ventilation
Aerosol therapy during non-invasive ventilation (within the circuit) was not used consistently, with half of respondents reporting this practice as usual (175, 20 %) or frequent (252, 29 %) and the other half reporting it as exceptional (148, 17 %) or never performed (279, 33 %). The primary reason for delivering aerosols during non-invasive ventilation was the severity of the patient’s condition preventing ventilation interruption (according to 52 % of the 575 respondents practicing aerosol therapy during non-invasive ventilation).
Of note, analyzing the subgroup of respondents (121) reporting frequent use of aerosolized antibiotics during MV (several patients a month), and who may be considered as more experienced, the results were similar to those of the rest of the population (see Electronic Supplementary Material). Analyzing other respondent subgroups (pediatricians or neonatologists, respondents working outside Europe) yielded results similar to those of the whole population (data not shown).
Knowledge and opinions
Almost all respondents (773, 90 %) considered aerosol therapy during MV of some interest (only 5 % had no opinion). The relationship between droplet size and the proximal to distal deposit ratio seemed familiar, but most respondents failed to answer more specific questions about optimal droplet size or nebulization yield (Fig. 4). Knowledge about the performance of specific nebulizer types was also poor, especially for vibrating mesh nebulizers (Electronic Supplementary Material Fig. E1).
A majority of respondents (73 and 72 %, respectively) considered that inhaled antimicrobial therapy can increase the effectiveness of pneumonia treatment (on top of intravenous antimicrobial therapy) and its tolerability (nebulized rather than intravenous aminoglycosides). Indeed, nebulized colistin was frequently (84 %) considered as an interesting therapeutic option in the treatment of pneumonia due to multidrug-resistant bacteria, but most respondents considered combination with an intravenous antibiotic necessary (Electronic Supplementary Material Fig. E2).
Discussion
The present study investigated current practice of aerosol therapy during MV among a large international panel of intensive care physicians. Despite surprisingly frequent use for some indications such as antibiotic therapy, and positive beliefs concerning the efficacy of this technique, physicians’ knowledge appeared limited on specific issues regarding aerosol delivery and efficacy, and was far short of current scientific knowledge.
Aerosolization, a frequent practice
Almost all respondents reported delivering aerosolized drugs during MV, especially, as expected, bronchodilators and to a lesser extent steroids (Fig. 2). Surprisingly, several antibiotics, in particular colistin, were reported as relatively frequently nebulized (usual practice according to 20 % and frequent practice according to 10 % of respondents; Fig. 2), although the benefit of this nebulization is debated [22–26]. Of note, our study revealed a positive belief towards the efficacy of inhaled antibiotics in enhancing the efficacy of intravenous treatment (75 % of respondents) or even as the cornerstone of the therapy in specific cases, despite a lack of strong clinical evidence [26]. Furthermore, numerous other drugs (sometimes off-label) were reported to be delivered as an aerosol (Table 1).
Despite frequent use of aerosols during MV, however, the devices and techniques used for aerosol delivery were far short of best available practice and some potentially dangerous practices were reported.
Suboptimal delivery techniques
In recent decades, a great number of studies have defined some settings allowing maximization of the nebulization yield during MV (Fig. 1). Briefly, various authors have found that changing ventilator settings (decreasing inspiratory flow and increasing inspiratory time), placing the nebulizer 10–30 cm upstream on the inspiratory limb and avoiding gas humidification allows the nebulization yield to be increased [6–11]. In the present study, the vast majority of respondents did not implement these principles in their clinical practice; e.g. only 12 % of respondents reported placing the nebulizer upstream on the inspiratory limb (Fig. 3), and 78 % of respondents did not avoid humidification during nebulization. Of note, these optimization principles may be less important for bronchodilator delivery since the efficacy of these drugs is high even when use is suboptimal [27, 28]. For example, several ventilator setting manipulations are not associated with improved efficacy of bronchodilators delivered through a metered dose inhaler [29, 30]. However, nebulization practice was similar in the subgroup of respondents who reported frequent use of aerosolized antibiotics, a situation in which optimization of the nebulization yield may be critical [14, 17]. This may illustrate the potential gap between experience and expertise [31].
Aside from these technical principles, respondents seemed not to have fully integrated into their practice recent technological advances concerning aerosol therapy during MV. Indeed, although a majority of respondents considered ultrasonic nebulizers superior to jet nebulizers, only half of them actually used them. The use of more recently developed vibrating mesh nebulizers, which are specifically designed for use during MV, was marginal (14 %). One can hypothesize that cost may be a factor limiting the use of these devices. Furthermore, although bronchodilators and steroids (the most frequently inhaled drugs) are available as metered dose inhalers, with abundant efficacy data for these devices [27, 32–34], only half of respondents (56 %) reported using them, often inadequately (patient disconnection and delivery into the tracheal tube) [12]. Technical issues and availability of equipment may partly explain the apparently limited use of an optimal technique.
Potentially hazardous practices
Jet nebulizers were the most frequently used devices, mostly within ventilator integrated systems allowing control of the delivered tidal volume during nebulization. Despite the availability of such systems, 14 % of respondents still reported connecting the jet nebulizer to an external gas source, thus exposing the patient to an uncontrolled tidal volume. When using a ventilator that was not comprising such an integrated system, 40 % of respondents acknowledged this potentially dangerous practice when using another aerosolization device (metered dose inhaler, ultrasonic or vibrating mesh nebulizer) would appear safer.
Another potential hazard associated with aerosol therapy during MV is related to exhaled particles. Indeed, during nebulization, a significant amount of droplets is cleared through the expiratory limb of the ventilator circuit. As these droplets may damage the expiratory flow meter of the ventilator, it is advisable to protect it by placing a filter on the expiratory limb (before the flow meter) [11, 35]. This practice was reported by only 65 % of respondents (Fig. 3). Furthermore, as exhaled particles impact on the protective filter, expiratory resistance may increase and complete filter obstruction has been reported with dramatic consequences [17, 36]. One-third of respondents using such protective filters reported never changing them, thus exposing patients to potentially severe complications.
Implications
The discrepancy observed between a frequent use on the one hand and suboptimal implementation and potentially hazardous practices on the other hand calls for action at educational and scientific levels.
First, general guidelines for aerosol therapy may address the issue of potentially hazardous practices [37, 38], and intensive care medicine societies may produce specific guidelines regarding aerosol therapy during MV. Concerning the complex implementation in daily practice of advances in the understanding of optimal aerosol therapy during MV, solutions are not so straightforward. The present study highlights some knowledge gaps among respondents, e.g. overestimation of nebulization yield, lack of knowledge about vibrating mesh nebulizers, ultrasonic nebulizers believed to produce smaller droplets and to outperform jet nebulizers. Those issues may be addressed through educational programs and guidelines. Nevertheless, some other results highlight the impracticability of transferring experimental results in the field of aerosol science into clinical practice. For instance, the fact that a majority of physicians did not change ventilator settings during nebulization may be due to a lack of knowledge, but the fact that among those who declared changing ventilator settings, 16 % restricted this practice to deeply sedated patients and 38 % sometimes used sedatives or muscle relaxants to do so illustrates that, although shown to be effective at the experimental level, ventilator setting changes are difficult to implement in clinical practice. Thus, experimental aerosol studies should focus on means of optimizing nebulization in ways as simple as possible to translate into daily clinical practice.
Limitations
As the present study was designed as an electronic survey, there are important limitations concerning data interpretation. First, respondents may have presented an inaccurate representation of the real practice of nebulization as performed in their department even though they provided answers they believed to be correct. Second, although the survey was anonymous, respondents may have tended to give responses in accordance with the literature rather than describing their real practice and beliefs. This potential bias would have tended to reduce discrepancies between responses and scientific evidence on the topic, and thus actual practice may be even further away from best scientific evidence concerning optimal nebulization technique during MV. A prospective observational study recording actual practice in intensive care units may allow these limitations to be overcome.
Despite the international scope of the study, a large majority of respondents worked in Europe and specifically in France, remaining respondents being distributed among numerous countries around the world. Therefore, even if the same response pattern was observed in these subgroups, the results may only partially be generalized to North American, Asian or African settings of intensive care medicine. The same limitation applies to the pediatric and neonatology setting. Last, although many respondents took part in the study, the overall response rate was relatively low, thus limiting the generalizability of the results.
Conclusions
The use of aerosol therapy during MV appeared to be frequent for the delivery of bronchodilators and steroids and also to a lesser extent for the delivery of antibiotics. Scientific knowledge about optimal implementation of aerosol therapy during MV seemed to be applied infrequently and the use of some potentially dangerous practices was reported. These issues may be addressed through educational programs and research focusing on simplifying bench-to-bedside transfer of knowledge.
References
Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176:532–555
Ramsey BW, Dorkin HL, Eisenberg JD, Gibson RL, Harwood IR, Kravitz RM, Schidlow DV, Wilmott RW, Astley SJ, McBurnie MA (1993) Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med 328:1740–1746
Hess D (1990) The delivery of aerosolized bronchodilator to mechanically ventilated intubated adult patients. Respir Care 35:399–404
Fuller HD, Dolovich MB, Posmituck G, Pack WW, Newhouse MT (1990) Pressurized aerosol versus jet aerosol delivery to mechanically ventilated patients. Comparison of dose to the lungs. Am Rev Respir Dis 141:440–444
MacIntyre NR, Silver RM, Miller CW, Schuler F, Coleman RE (1985) Aerosol delivery in intubated, mechanically ventilated patients. Crit Care Med 13:81–84
O’Riordan TG, Greco MJ, Perry RJ, Smaldone GC (1992) Nebulizer function during mechanical ventilation. Am Rev Respir Dis 145:1117–1122
Diot P, Morra L, Smaldone GC (1995) Albuterol delivery in a model of mechanical ventilation. Comparison of metered-dose inhaler and nebulizer efficiency. Am J Respir Crit Care Med 152:1391–1394
Fink JB, Dhand R, Grychowski J, Fahey PJ, Tobin MJ (1999) Reconciling in vitro and in vivo measurements of aerosol delivery from a metered-dose inhaler during mechanical ventilation and defining efficiency-enhancing factors. Am J Respir Crit Care Med 159:63–68
Miller DD, Amin MM, Palmer LB, Shah AR, Smaldone GC (2003) Aerosol delivery and modern mechanical ventilation: in vitro/in vivo evaluation. Am J Respir Crit Care Med 168:1205–1209
Dhand R (2008) Aerosol delivery during mechanical ventilation: from basic techniques to new devices. J Aerosol Med Pulm Drug Deliv 21:45–60
Guerin C, Fassier T, Bayle F, Lemasson S, Richard JC (2008) Inhaled bronchodilator administration during mechanical ventilation: how to optimize it, and for which clinical benefit? J Aerosol Med Pulm Drug Deliv 21:85–96
Rau JL, Harwood RJ, Groff JL (1992) Evaluation of a reservoir device for metered-dose bronchodilator delivery to intubated adults. An in vitro study. Chest 102:924–930
Goldstein I, Wallet F, Robert J, Becquemin MH, Marquette CH, Rouby JJ (2002) Lung tissue concentrations of nebulized amikacin during mechanical ventilation in piglets with healthy lungs. Am J Respir Crit Care Med 165:171–175
Goldstein I, Wallet F, Nicolas-Robin A, Ferrari F, Marquette CH, Rouby JJ (2002) Lung deposition and efficiency of nebulized amikacin during Escherichia coli pneumonia in ventilated piglets. Am J Respir Crit Care Med 166:1375–1381
Palmer LB, Smaldone GC, Chen JJ, Baram D, Duan T, Monteforte M, Varela M, Tempone AK, O’Riordan T, Daroowalla F, Richman P (2008) Aerosolized antibiotics and ventilator-associated tracheobronchitis in the intensive care unit. Crit Care Med 36:2008–2013
Ehrmann S, Mercier E, Vecellio L, Ternant D, Paintaud G, Dequin PF (2008) Pharmacokinetics of high-dose nebulized amikacin in mechanically ventilated healthy subjects. Intensive Care Med 34:755–762
Lu Q, Yang J, Liu Z, Gutierrez C, Aymard G, Rouby JJ (2011) Nebulized ceftazidime and amikacin in ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med 184:106–115
Dhand R (2004) New frontiers in aerosol delivery during mechanical ventilation. Respir Care 49:666–677
Dhand R (2012) Aerosol therapy in patients receiving noninvasive positive pressure ventilation. J Aerosol Med Pulm Drug Deliv 25:63–78
Plaza V, Sanchis J, Roura P, Molina J, Calle M, Quirce S, Viejo JL, Caballero F, Murio C (2012) Physicians’ knowledge of inhaler devices and inhalation techniques remains poor in Spain. J Aerosol Med Pulm Drug Deliv 25:16–22
Burns KEA, Duffett M, Kho ME, Meade MO, Adhikari NKJ, Sinuff T, Cook DJ (2008) A guide for the design and conduct of self-administered surveys of clinicians. CMAJ 179:245–252
Kofteridis DP, Alexopoulou C, Valachis A, Maraki S, Dimopoulou D, Georgopoulos D, Samonis G (2010) Aerosolized plus intravenous colistin versus intravenous colistin alone for the treatment of ventilator-associated pneumonia: a matched case-control study. Clin Infect Dis 51:1238–1244
Rattanaumpawan P, Lorsutthitham J, Ungprasert P, Angkasekwinai N, Thamlikitkul V (2010) Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J Antimicrob Chemother 65:2645–2649
Kwa AL, Loh C, Low J, Kurup A, Tam VH (2005) Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis 41:754–757
Kwa AL, Falagas ME, Michalopoulos A, Tam VH (2011) Benefits of aerosolized colistin for ventilator-associated pneumonia: absence of proof versus proof of absence? Clin Infect Dis 52:1278–1279
Linden PK, Paterson DL (2006) Parenteral and inhaled colistin for treatment of ventilator-associated pneumonia. Clin Infect Dis 43:S89–S94
Dhand R, Duarte AG, Jubran A, Jenne JW, Fink JB, Fahey PJ, Tobin MJ (1996) Dose-response to bronchodilator delivered by metered-dose inhaler in ventilator-supported patients. Am J Respir Crit Care Med 154:388–393
Bernasconi M, Brandolese R, Poggi R, Manzin E, Rossi A (1990) Dose-response effects and time course of effects of inhaled fenoterol on respiratory mechanics and arterial oxygen tension in mechanically ventilated patients with chronic airflow obstruction. Intensive Care Med 16:108–114
Mouloudi E, Katsanoulas K, Anastasaki M, Hoing S, Georgopoulos D (1999) Bronchodilator delivery by metered-dose inhaler in mechanically ventilated COPD patients: influence of tidal volume. Intensive Care Med 25:1215–1221
Mouloudi E, Prinianakis G, Kondili E, Georgopoulos D (2001) Effect of inspiratory flow rate on beta2-agonist induced bronchodilation in mechanically ventilated COPD patients. Intensive Care Med 27:42–46
Choudhry NK, Fletcher RH, Soumerai SB (2005) Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med 142:260–273
Malliotakis P, Linardakis M, Gavriilidis G, Georgopoulos D (2008) Duration of salmeterol-induced bronchodilation in mechanically ventilated chronic obstructive pulmonary disease patients: a prospective clinical study. Crit Care 12:R140
Guerin C, Chevre A, Dessirier P, Poncet T, Becquemin MH, Dequin PF, Le Guellec C, Jacques D, Fournier G (1999) Inhaled fenoterol-ipratropium bromide in mechanically ventilated patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159:1036–1042
Dhand R, Tobin MJ (1996) Bronchodilator delivery with metered-dose inhalers in mechanically-ventilated patients. Eur Respir J 9:585–595
Mercier E, Dequin PF, Vecellio L (2008) Aerosols during mechanical ventilation. Rev Mal Respir 25:731–741
Davies JBS, Bromilow J (2011) Bacterial filter obstruction with the use of ultrasonic nebulisation. Anaesthesia 66:394–395
Laube BL, Janssens HM, de Jongh FHC, Devadason SG, Dhand R, Diot P, Everard ML, Horvath I, Navalesi P, Voshaar T, Chrystyn H (2011) ERS/ISAM task force consensus statement. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 37:1308–1331
Dolovich MA, MacIntyre NR, Anderson PJ, Camargo CA, Chew N, Cole CH, Dhand R, Fink JB, Gross NJ, Hess DR, Hickey AJ, Kim CS, Martonen TB, Pierson DJ, Rubin BK, Smaldone GC (2000) Consensus statement: aerosols and delivery devices. American Association for Respiratory Care. Respir Care 45:589–596
Acknowledgments
The authors sincerely thank Mrs. Dominique De Boom of the European Critical Care Research Network and Adrien Constan of the REVA network for their valuable help in the conduct of this study. The authors affiliated with Equipe Biomécanique Cellulaire et Respiratoire acknowledge receipt of a grant from Agence Nationale de la Recherche (ANR-2010 BLAN 1119 05).
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ehrmann, S., Roche-Campo, F., Sferrazza Papa, G.F. et al. Aerosol therapy during mechanical ventilation: an international survey. Intensive Care Med 39, 1048–1056 (2013). https://doi.org/10.1007/s00134-013-2872-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2872-5