Article Text
Statistics from Altmetric.com
Dr Andrew W Creamer (AWC), Respiratory Registrar: A 70-year-old retired teacher was referred to the respiratory clinic with a 12-month history of progressive exertional dyspnoea and dry cough. He denied haemoptysis, constitutional symptoms or diurnal variation. He took omeprazole, amlodipine and ezetimibe for a long-standing history of dyspepsia, hypertension and hypercholesterolaemia. He was an ex-smoker with a 30 pack-year history.
He reported monophasic colour change in his fingers in the cold, but denied any other connective tissue disease (CTD) symptoms. He denied any significant environmental exposures such as organic dusts or moulds.
His vital signs were normal. There was evidence of mechanic’s hands (a hyperkeratotic eruption with fissuring and cracking on the palmar and radial aspects of the fingers, strongly associated with idiopathic inflammatory myopathies) but no other features of CTD or clubbing. Bibasal fine inspiratory crepitations were audible on chest auscultation. Cardiovascular examination was normal.
Initial investigations revealed pancytopenia (figure 1) with unremarkable liver and renal function, prompting referral to local haematology services for further assessment.
Baseline blood tests at presentation. Abnormal values are highlighted in bold. Pancytopenia was evident, in the context of normal B12, folate and ferritin.
Lung function testing revealed a restrictive pattern with significantly reduced gas transfer (figure 2), while 6 min walk test demonstrated baseline saturations of 95% on air dropping to 77% on exertion, achieving 260 m (48% of theoretical distance).
Lung function tests at baseline and 4-month follow-up. Spirometry demonstrated a restrictive pattern with reduction in gas transfer factor. KCO, carbon monoxide transfer coefficient; TLCO, transfer factor for carbon monoxide.
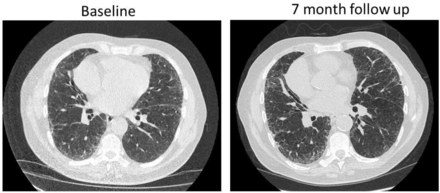
Chest X-ray showed prominent interstitial lung markings in the mid and lower zones (figure 3). High-resolution CT thorax (HRCT) demonstrated centrilobular emphysema throughout the lungs and a basally predominant interstitial process with features suggestive of non-specific interstitial pneumonia (NSIP) (figure 4). Transthoracic echocardiogram showed preserved left ventricular systolic function with mild concentric hypertrophy and normal right ventricular size and function. There was no tricuspid regurgitation, so an estimate of the pulmonary artery pressure could not be made.
Erect chest X-ray demonstrating prominent interstitial lung markings, particularly in the mid and lower zones.
High-resolution CT thorax at baseline and 7-month follow-up. The initial CT demonstrates centrilobular emphysema throughout the lungs and a basally predominant interstitial process with subpleural reticulation and ground glass opacification. On multidisciplinary team review this was felt to be consistent with a non-specific interstitial pneumonia. Repeat CT at 7 months demonstrates progression in reticulation and ground glass.
Dr Shaney L Barratt (SLB), Respiratory Consultant: The differential diagnosis for interstitial lung disease (ILD) presenting in a 70-year-old is broad and includes hypersensitivity pneumonitis, drug-induced ILD, connective tissue disease-associated ILD (CTD-ILD) and idiopathic interstitial pneumonias, particularly idiopathic pulmonary fibrosis. There was no history of pneumotoxic medication and no relevant occupational exposures. The radiological pattern and absence of relevant exposures made hypersensitivity pneumonitis less likely. The presence of mechanic’s hands in a patient with an NSIP pattern suggests the possibility of an underlying CTD, and an autoimmune screen is an important part of the initial assessment of new patients with ILD.
The combination of pancytopenia with ILD should prompt consideration of a short telomere syndrome, particularly if there is a family history of pulmonary fibrosis or bone marrow failure. These are a heterogeneous group of inherited disorders of variable penetrance caused by mutations that interfere with the normal maintenance of telomeres. However, the patient’s age, absence of family history, and lack of typical skin or systemic features would not support this.
Given the wide differential and thorough diagnostic work-up required, a multidisciplinary team (MDT) diagnosis taking into consideration clinical, radiological, histopathological and serological features is essential.
AWC: Indirect immunofluorescence using HEp-2 cells demonstrated a cytoplasmic speckled pattern with no nuclear staining (antinuclear antibody-negative/anticytoplasmic antibody-positive). Subsequent myositis spectrum antibody immunoblot testing confirmed anti-threonyl-tRNA synthetase (anti-PL-7) antibodies (figure 5).
Myositis immunoblot (Euroline, Germany) demonstrating positive ++ staining of the anti-threonyl-tRNA synthetase (anti-PL-7) antibody. The manufacturer instructs that (+) results should be considered negative. Co: positive control +++. OJ/EJ/PL12 and so on represent alternative myositis-associated antibodies, which were negative.
In the context of his clinical features and anti-PL-7 positivity, a diagnosis of lung-dominant antisynthetase syndrome (ASyS) was made. The patient was commenced on prednisolone 20 mg once daily, with co-trimoxazole prophylaxis against opportunistic infection, and referred for assessment for ambulatory oxygen therapy, pending haematological review.
Dr Rachel Protheroe (RP), Haematology Consultant: Bone marrow biopsy demonstrated dysplastic megakaryocytes and erythrocytes, reduced granulopoiesis and myeloid blasts (6%), consistent with a diagnosis of myelodysplastic syndrome (MDS) with excess blasts.
MDS is a clonal haematopoietic stem cell disorder characterised by dysplastic, ineffective haematopoiesis associated with progression to acute myeloid leukaemia (AML). Treatment is indicated according to the International Prognostic Scoring System,1 in this case an intermediate 2 risk. Such higher risk MDS carries a major risk of transformation to AML and in fit patients can be treated with intensive chemotherapy consolidated with allogeneic stem cell transplantation, but the severity of the ILD precluded this. He was therefore treated with azacitidine, a hypomethylating agent.
There is a recognised association between MDS and autoimmune phenomena (AIP), with a variety of serological and clinical syndromes of autoimmunity reported in patients with MDS. Given the temporal relationship between the development of MDS and ASyS-associated ILD, it was felt that the two may be linked.
AWC: After five cycles of azacitabine, a good response in the peripheral blood count was seen (Hb 88 g/L, white cell count 1.52x109/L , platelets 227x109/L). Unfortunately, lung function continued to deteriorate (figure 2) with worsening dyspnoea. HRCT showed progression of the ground glass component of the ILD (figure 4). Mycophenolate Mofetil 500 mg once daily was commenced, but had to be stopped after four doses due to fall in neutrophils to 0.17×109/L. A further trial of prednisolone at a higher dose of 30 mg was started without significant benefit. In view of the progressive disease despite maximum tolerable immunosuppressive therapy, a pathway of low-dose prednisolone, alongside best supportive care, was adopted.
AWC, Dr Harsha Gunawardena (HG), Rheumatology Consultant, RP and SLB: We present a case of ASyS-associated ILD with a synchronous diagnosis of MDS, with the simultaneous presentation suggesting a causative relationship. To the authors’ knowledge, this is the first described case of ASyS developing in MDS and of a CTD-ILD associated with MDS.
ASyS is a distinct subset of autoimmune CTD, which is historically described within the idiopathic inflammatory myopathy spectrum.2 It is characterised by the presence of autoantibodies against one of several cytoplasmic aminoacyl transfer RNA synthetase enzymes.2 It is most frequently associated with anti-Jo-1, although seven other autoantibodies, including anti-PL-7, have been identified.3 Clinical presentation may phenotypically overlap with dermatomyositis (DM) or polymyositis, but ASyS can exist in an amyopathic form.4 Other clinical features include inflammatory arthritis, myositis, mechanic’s hands, fever and Raynaud’s. Associated ILD is common, with lung involvement present in 50%–90% cases depending on the autoantibody expressed.5 In published studies of anti-PL-7-associated ASyS, pulmonary involvement occurred in 66%–100%.3 6 The most common pattern of lung involvement is NSIP, with organising pneumonia occurring in a minority of cases.6
Management of ASyS-associated ILD involves pharmacological and non-pharmacological measures. As in other chronic respiratory conditions, oxygen therapy, vaccination and smoking cessation should be addressed. Pulmonary rehabilitation should be encouraged for patients with myositis-associated ILD; studies have demonstrated that physical exercise is safe in myositis and improves muscle strength,7 although rehabilitation programmes should be delayed if there is evidence of active muscle inflammation. Immunomodulatory agents form the mainstay of pharmacological treatment of ASyS-ILD. No specific guidelines exist, and the approach should be tailored to the acuteness and severity of the initial presentation. A proposed algorithm was published recently,8 which suggested that in patients with stable mild-moderate disease, oral prednisolone should be started alongside a steroid-sparing agent (Mycophenolate Mofetil or azathioprine). If there is improvement or stabilisation, the steroid can be weaned down. In patients presenting acutely or in respiratory compromise, high-dose steroids (typically pulsed intravenous methylprednisolone) should be started in combination with ciclosporin or a calcineurin inhibitor. Rituximab, an anti-CD20 monoclonal antibody, has been used in refractory cases with some success.9 The proposal suggests that in treatment failure, a trial of intravenous immunoglobulin or referral for transplantation is considered.
There is a recognised association between MDS and AIP. A recent large-scale multicentre study of 1408 patients with MDS found evidence of an autoimmune process in 28%,10 in keeping with previous studies. In this cohort, the presence of AIPs was associated with improved overall survival (60 months vs 45 months) and a lower chance of transformation to acute leukaemia (23% vs 30%). A wide range of AIPs have been reported in MDS, with systemic conditions such as Sjogren’s syndrome, systemic lupus erythematosus and systemic vasculitides all described. Serological evidence of autoimmunity is frequently identified.11 There are a small number of case reports of DM (with lung involvement) associated with MDS,12 including melanoma differentiation-associated protein 5 (MDA-5)-associated DM.13 The underlying pathogenic mechanisms for the association between MDS and autoimmune processes are unknown, although deranged cytokine production, as well as more specific changes in T-cell responses to antigen presentation and abnormal B-cell and T-cell interactions, have been proposed. In published series, immunosuppressive therapy has improved the AIP and haematological indices in a proportion of cases, but this effect is not consistent.14 As in the case outlined here, management of these cases is often challenging,12 and treatment approaches must reflect both the haematological and systemic pathologies.
A distinct association between ILD and MDS has also been described. In a cohort study of 827 patients with MDS, 2% had ILD, a rate significantly higher than that of the general population.15 In this study, only 1 of the 18 patients with ILD had a CTD diagnosis (rheumatoid arthritis), and none had received a hypomethylating agent prior to diagnosis of ILD. Telomere dysfunction was proposed as a potential shared mechanism, but further research is required to elucidate the exact pathophysiology.
This case describes a lung-dominant ASyS developing in the context of MDS, presenting with ILD and bone marrow failure. An important alternative diagnosis to consider in the combination of bone marrow failure and lung fibrosis is telomere-related disease. Telomeres are highly conserved 5’-TTAGGG-3’ repeats at the ends of chromosomes that confer protection during cellular replication. At a molecular level, telomere dysfunction triggers a DNA damage response cascade that results in cellular senescence and apoptosis that may be cell type-specific or tissue type-specific. The clinical manifestations of telomere-mediated disease are thus diverse, although the triad of bone marrow failure, liver cirrhosis and idiopathic pulmonary fibrosis is considered to be highly predictive of germline telomere mutation.16 Six telomere-related genes have been linked to the development of familial pulmonary fibrosis, although TERT (telomerase reverse transcriptase) mutations are most frequent, occurring in approximately 15% of those with affected kindreds.17 If suspected, telomere length analysis or genetic analysis of telomerase-related genes (TERT, TERC, DKC1, TINF2, PARN and RTEL1) could be performed.
This case raises several important points for clinicians. ILD is a feature of many CTDs, and as in this amyopathic presentation, the classic musculocutaneous features of the condition may be subtle or absent. New ILD presentations should therefore prompt a thorough evaluation for symptoms and signs of a CTD. As a minimum we suggest this should include a detailed history exploring arthralgia, myalgia, skin changes, and presence of Raynaud’s or sicca symptoms, with careful examination of the hands for subtle clinical signs of mechanic’s hands, fingertip ulceration, rugged cuticles, dilated nailfold capillaries, finger tapering, Gottron’s papules, and heliotrope rashes of the face, posterior neck and shoulders (shawl sign) or of the anterior neck and chest (V-sign). A basic laboratory screen of anti-nuclear antibodies (ANA), rheumatoid factor, anticyclic citrullinated peptide and creatine kinase (CK) is recommended. In the context of suspected ASyS, a negative ANA screen does not necessarily indicate autoantibody negativity; the presence of cytoplasmic staining using cell immunofluorescence would be characteristic. We suggest full immunoblot for myositis-specific antibodies (anti: Jo-1, PL-12, PL-7, KS, OJ, EJ, ZO, Ha and MDA-5) and myositis-associated antibodies in those with muscle complaints, unexplained elevated CK or clinical signs such as mechanic’s hands. Second, given the recognised association between MDS and ILD and autoimmune conditions, the presence of an unexplained cytopaenia should prompt referral to a haematologist. Finally, this case demonstrates the complex, multisystemic nature of CTD-ILD, requiring the input of respiratory and rheumatology physicians with MDT support. A specialist-combined respiratory/rheumatology ILD-CTD clinic, such as that which runs at our centre, helps to streamline this process.
References
Footnotes
Contributors AWC: first author, wrote the case outline, wrote the final version of the article. RP: contributed to the section on myelodysplastic syndrome, and reviewed and edited the final version of the text. HG: contributed to the section on antisynthetase syndrome/connective tissue disease-associated ILD, and reviewed and edited the final version of the text. SLB: wrote the section on assessment of ILD (under her name in the text), reviewed and edited the final version of the text, and is responsible for the overall content as guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.