Article Text
Abstract
Background Epithelial to mesenchymal transition (EMT) is associated with the pathophysiology of chronic rhinosinusitis with nasal polyp (CRSwNP). Wnt signaling is causative for EMT, whereas the mechanism in CRSwNP is not fully understood.
Objective We sought to evaluate the role of Wnt signaling in EMT of CRSwNP using a murine nasal polyp (NP) model and human tissues.
Methods Inflammatory markers and EMT-related molecules were evaluated in NP models using adenomatosis polyposis coli (Apc)Min/+ mice with activated Wnt signaling and NP models treated with Wnt signaling inhibitor, indocyanine green-001 (ICG-001). EMT markers and Wnt signaling-associated mediators were analysed using human sinonasal tissues from control subjects and CRSwNP patients.
Results ApcMin/+ mice-induced NPs exhibited more frequent polypoid lesions and upregulation of Wnt-related molecules, including nuclear β-catenin, WNT3A and cyclin D1. Markers of EMT were significantly overexpressed in the ApcMin/+ NP mice (p<0.001 for E-cadherin and α-smooth muscle actin), and interleukin (IL)-17A+ cells and neutrophilic infiltration were increased in ApcMin/+ NP mice (p<0.001). Inhibition of Wnt signaling via ICG-001 resulted in significantly decreased nasal polypoid lesions (p<0.001), EMT-related markers (p=0.019 for E-cadherin and p=0.002 for vimentin) and the mRNA levels of IL-4 (p<0.001) and IL-17A (p=0.004) compared with the positive control group. Finally, nuclear β-catenin (p=0.042) was significantly increased compared with the control, and the expression levels of Wnt ligands and receptors were upregulated in human NP tissues (p=0.045 for WNT3A and p=0.042 for FZD2), suggesting increased Wnt signaling and EMT in CRSwNP.
Conclusion Wnt signaling may contribute to the pathogenesis of NPs through EMT. Therefore, inhibition of Wnt signaling may be a potential therapeutic strategy for patients with CRSwNP.
- airway epithelium
- cytokine biology
- histology/cytology
- innate immunity
Statistics from Altmetric.com
Key messages
What is the key question?
Does Wnt signaling affect nasal polypogenesis through epithelial to mesenchymal transition (EMT) in the sinonasal epithelium and can epithelial changes be restored via inhibition of Wnt signaling?
What is the bottom line?
Aberrant Wnt signaling affected nasal polypogenesis with tissue remodelling and EMT and increased sinonasal airway inflammation. Inhibition of Wnt signaling with indocyanine green-001, a selective β-catenin/CBP inhibitor, effectively reduced nasal polyp (NP) formation and the inflammatory burden at levels similar to that observed with dexamethasone treatment in a murine NP model.
Why read on?
Wnt-induced EMT was related to the pathogenesis of NPs in a murine model using adenomatosis polyposis coli mutant mice and human sinonasal tissues from patients with chronic rhinosinusitis with nasal polyp (CRSwNP). Wnt signaling may be a possible therapeutic target for patients with CRSwNP.
Introduction
Nasal polyps (NPs) arising from the sinonasal mucosa are caused by chronic inflammation and tissue remodelling and are characterised by swelling, fluid collection and increased fibrin deposition.1 The disease status of epithelial lining in the paranasal sinuses can lead to blockage of the natural ostium and nasal passage. Therefore, patients with chronic rhinosinusitis with nasal polyp (CRSwNP) present typical symptoms, including nasal obstruction, rhinorrhoea, hyposmia and sinus pressure.2 Among all of the potential pathophysiological mechanisms, it has been documented that epithelial barrier disruption of the sinonasal mucosa is associated with CRSwNP.3–5 However, the cellular signaling mechanisms associated with NP formation have not been fully elucidated.
Epithelial to mesenchymal transition (EMT) has been reported to participate in the polypogenesis of CRS.6 7 EMT, initially recognised in embryogenesis, is the central mechanism of wound healing and tissue fibrosis.8 9 EMT is a process in which epithelial cells lose polarity, junctional proteins are downregulated, the cytoskeleton becomes reorganised, and mesenchymal cell phenotypes are obtained.10 Besides, loss of the epithelial adhesion molecule, E-cadherin, is a typical finding during EMT; this phenomenon has been observed in CRSwNP tissues.7 A state of chronic inflammation in patients with CRSwNP, especially those who have eosinophilic infiltration, may induce EMT, and a subsequent persistent EMT process may accelerate NP formation.11 When E-cadherin is attenuated after EMT, β-catenin is released and activates the Wingless/integrase-1 (Wnt) signaling pathway.12 A recent study indicates that the canonical Wnt/β-catenin signaling pathway is activated in CRSwNP, and Wnt signaling can alter the epithelial morphology of nasal mucosa and lead to decrease cell to cell adhesion.13 Thus, dysregulated activation of the Wnt signaling pathway has been shown to be causative for EMT, and Wnt-induced EMT has been linked to progression and metastasis of cancers.14–16 Furthermore, aberrant Wnt signaling and EMT has been implicated in chronic lower airway diseases, including asthma, chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis, and Wnt signaling play a role in angiogenesis and tissue remodelling of lung tissues.17 18 Transforming growth factor (TGF)-β1 via Smad3 signaling pathway is also involved in EMT.9 In previous studies on lower airway diseases such as asthma or COPD, TGF-β1 and Smad3 were known to induce EMT in bronchial mucosa.19 20 In human nasal epithelial cells (hNECs), TGF-β1 can promote EMT via upregulation of α-smooth muscle actin (α-SMA).6 Furthermore, crosstalk between TGF-β1 and Wnt signaling pathway has been reported.
In the present study, we sought to evaluate the role of Wnt signaling on the EMT in nasal polypogenesis. Specifically, we first used a murine NP model with adenomatosis polyposis coli (Apc) mutant mice (ApcMin/+ mice), which has an aberrant activation of Wnt signaling and was generated by random Apc mutations.21 22 After that, we evaluated the effect of indocyanine green-001 (ICG-001), an inhibitor of β-catenin/cyclic adenosine monophosphate-response element-binding protein-binding protein (CBP), and finally analysed molecular markers associated with remodelling and EMT in human sinonasal tissues from CRSwNP patients.
Methods
A murine NP model using ApcMin/+ mice
Nasal polypoid lesions in mice were induced according to a previously established protocol.23 24 Briefly, mice were sensitised with ovalbumin (OVA) in aluminium hydroxide gel intraperitoneally, followed by intranasal instillation of OVA for 12 consecutive weeks. During the last eight straight weeks, staphylococcal enterotoxin B (SEB) was challenged via the nostril. These animals were maintained in a specific pathogen-free biohazard containment facility. All of the animal experiments were conducted following the guidelines and ethics of Institutional Animal Care at the Clinical Research Institute of Dankook University Hospital (No. DKU-16–027). C57BL/6J and ApcMin/+ mice were divided into four groups (n=20); (1) wild-type control group, instilled with phosphate-buffered saline (PBS), (2) wild type NP group, (3) ApcMin/+ PBS-instilled control group and (4) ApcMin/+ NP group. Littermate controls from ApcMin/+ mice were used as wild type mice. Detailed information is described in the online supplementary methods.
Supplemental material
Wnt signal inhibition in a murine model with NP
ICG-001 is known as a selective β-catenin/CBP inhibitor.25 ICG-001 was used to inhibit the Wnt signaling pathway and dexamethasone for the treatment of NP. Female BALB/c mice (4 weeks old, Orient Bio, Seongnam, South Korea) were used in the Wnt signal inhibition experiments and were divided into four groups (n=20); (1) PBS-instilled negative control group, (2) OVA/SEB-induced positive control NP group, (3) NP group treated with ICG-001 and (4) NP group treated with dexamethasone as conventional treatment of CRS. Spleen single-cell suspensions from negative control mice and positive control NP mice stimulated with OVA only or OVA/ICG-001, respectively. ELISA was performed to measure cytokines in splenic cell cultures treated with OVA and ICG-001, as previously described.26 Specifically, the levels of interleukin (IL)-4, IL-5, IL-17 and interferon (IFN)-γ in the culture supernatant were quantified using a sandwich ELISA kit (R&D Systems, Minneapolis, Minnesota, USA).
Histopathologic evaluation, immunohistochemistry and immunofluorescence
Polypoid lesions in our murine NP model were defined as distinct mucosal elevations with eosinophilic infiltration and microcavity formation.23 The number of polypoid lesions was enumerated by two independent examiners using H&E stained mucosal samples. Samples were also stained to compare specific characteristics using Sirius red for eosinophils and collagen deposition, periodic acid-Schiff (PAS) for goblet cells, and antineutrophilic antibodies for identification of neutrophils.
EMT and Wnt signaling markers, including E-cadherin, β-catenin, α-SMA, cyclin D1 and twist, were immunostained in paraffin sections of sinonasal tissues. Additional markers were evaluated using immunohistochemistry (IHC), such as Ki-67, acetyl-α-tubulin, TGF-β1 and phospho-Smad3 (p-Smad3). Signature cytokines such as IL-4, IL-17A and IFN-γ were evaluated with IHC using their primary antibodies (see online supplementary table S1).
Three serial coronal sections of each mouse (C57BL/6J, n=20 and BALB/c, n=20) were observed under a light microscope (high-power field; x400, BX-53; Olympus, Japan) by two blinded observers. The staining intensity of IHC was analysed using Image J software (National Institute of Health, Bethesda, Maryland, USA). The level of expression in IHC was measured using expression scores: zero for none, one for mild, two for moderate and three for strong expression. Both cellular count and expression scores were measured by three blinded observers. EMT markers and Wnt signaling molecules were assessed in the epithelial layer, and cell infiltration (eosinophils, neutrophils and IL-17A+ cells) were evaluated in the subepithelial layer. The expression of WNT3A and vimentin in the epithelial layer were assessed using immunofluorescence (IF) and examined under confocal microscopy (FV-3000, Olympus, Japan).
Quantitative real-time PCR and gene PCR array
Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s instructions. Equivalent amounts of RNA were reversed-transcribed using the iScript cDNA Synthesis Kit (Bio-red Laboratories; Hercules, California, USA). The mRNA expression analysis was performed using an Applied Biosystem 7500 Real-Time PCR System (Applied Biosystems, Foster City, California, USA). Relative expression levels of mRNA were measured for IL-4, IL-5, IL-17 and IFN-γ mouse genes. Also, mRNA expression levels of Wnt signaling molecules were analysed using PCR (WNT1, WNT2B, WNT3A, WNT5B, WNT8A and WNT10B). Wnt receptors such as Frizzled (FZD) 1, FZD2, FZD3 and β-catenin were also evaluated. Corresponding SYBR green primers and TaqMan probes were purchased from Bioneer (Daejeon, South Korea) and Applied Biosystems (see online supplementary tables S2,S3). The relative mRNA gene expression was calculated using the 2-ΔΔCt method and normalised with internal control GADPH. EMT-related gene PCR array was conducted using six samples (three wild type control mice and 3 ApcMin/+ control mice). The whole sinonasal mucosa of ApcMin/+ mice and its littermate was taken out meticulously using small curettes and microforceps under the microscopic vision. Both were neither PBS-instilled model nor OVA/SEB-induced NP model. Customised RT² Profiler PCR Arrays (Qiagen, Hilden, Germany) were used to analyse 96 mouse EMT-related markers, as previously described.27 For details, see online supplementary methods.
Measurement of IgE, IgG1, and IgG2a in mice serum
Serum levels of total IgE and OVA-specific IgE were quantified using solid-phase ELISA. OVA-specific IgG1 and IgG2a were analysed using biotinylated rat anti-mouse IgG1 (BD Pharmingen, San Jose, California, USA) and IgG2a (BD Pharmingen), respectively.
Patients and tissue samples
Sinonasal and polyp tissues were harvested from functional endoscopic sinus surgery in patients with CRSwNP. The diagnosis of CRSwNP was established based on the European position paper on rhinosinusitis and nasal polyps 2012 guideline.2 Patients were excluded who were younger than 18 years old, had diagnosed primary ciliary dyskinesia or had undergone prior treatment with antibiotics or corticosteroids or other immune-modulating drugs 4 weeks prior to surgery. Control tissues were harvested from patients without sinonasal disease who underwent skull base surgery or endoscopic dacryocystorhinostomy. We enrolled patients and harvested mucosal tissues from uncinate process (UP) of control subjects (n=15), UP of CRS without NP (CRSsNP, n=23), UP of CRSwNP (n=48) and NP of CRSwNP patients (n=44). Clinical data including Lund-Mackay scores, Lund-Kenney scores and 22-item SinoNasal Outcome Test (SNOT-22) were reviewed.28–30 Informed consent was obtained from all patients. Demographic data have been presented in table 1.
Demographics of subjects evaluated by histologic and molecular analyses
Culture of epithelial cell and Wnt stimulation, IF and immunoblotting
Normal hNECs (PromoCell, Heidelberg, Germany) were cultured under submerged condition. hNECs were cultured in 1% foetal bovine serum (FBS)-supplemented medium for 1 hour before recombinant human WNT3A (R&D Systems) stimulation. Cells were then stimulated with WNT3A (200 ng/mL) in fresh medium supplemented with 10% FBS for 48 hours. Fixed hNECs were incubated with primary antibodies and IF secondary antibodies. For immunoblotting, proteins were separated in 8%–12% sodium dodecyl sulfate–polyacrylamide gels, and transferred to Immobilon-P (Millipore, Billerica, Massachusetts, USA). Membranes were incubated sequentially with indicated primary and secondary antibodies; the antibodies used are specified in online supplementary table S4 (see the online supplementary methods).
Statistical analysis
Data were reported as median with IQR and were analysed by the Kruskal-Wallis test with Dunn multiple comparison test. Correlations were tested by Spearman’s rank correlation coefficients to assess the association between molecular markers and clinical parameters. A p<0.05 was considered statistically significant. Analyses were performed using Stata software V.14.0 (StataCorp, College Station, Texas, USA) and Graphpad prism software V.7.0 (GraphPad Software, La Jolla, California, USA).
Results
Wnt signaling and EMT are activated in ApcMin/+ mice with NPs
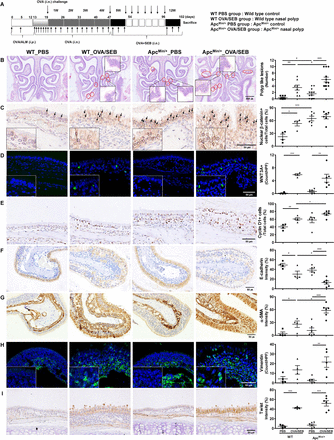
Murine NP models were established using ApcMin/+ mice and its wild-type littermate according to an established reported protocol (figure 1A).23 31 ApcMin/+ mice have been widely used as an intestinal tumorigenesis model.32–34 These transgenic mice have a predisposition for polypogenesis and overexpression of Wnt signaling. We, therefore, adapted ApcMin/+ mice for use in an NP model. ApcMin/+ NP mice exhibited more frequent polypoid lesions compared with the wild-type NP mice (p=0.032) and ApcMin/+ control group (p<0.001, figure 1B). Expression of nuclear β-catenin in the epithelial layer was generally increased in ApcMin/+ mice compared with the wild type (p<0.001), which reflects activation of Wnt/β-catenin signaling pathway in the ApcMin/+ mice (figure 1C). The expression of WNT3A in IF staining was upregulated in the NP-induced models (p<0.001, figure 1D) and cyclin D1, the target gene of Wnt/β-catenin signaling,35 was increased in the ApcMin/+ NP mice (p=0.034, figure 1E).
Increased Wnt signaling and epithelial to mesenchymal transition (EMT) in ApcMin/+ mice. (A) A protocol of the murine model of chronic rhinosinusitis (CRS) with NP in wild type (WT) C57BL/6J and ApcMin/+ mice. Ovalbumin (OVA) and staphylococcal enterotoxin B (SEB) were instilled into the nasal cavity to induce nasal polyposis. (B) Representative polypoid lesions which are indicated with red circles and the number of polypoid lesions. (C) Nuclear β-catenin-positive cell numbers were examined in a high-power field (HPF; x400). (D) WNT3A-positive cell numbers in immunofluorescence. (E) The number of cyclin D1-positive cells per total epithelial cells. (F) Immunohistochemical intensity of E-cadherin. (G) Immunohistochemistry of mesenchymal markers, α-smooth muscle actin (SMA). (H) Vimentin-positive cell counts using immunofluorescence. (I) The intensity of twist as a transcriptional repressor of E-cadherin gene. *P<0.05, **P<0.01, ***P<0.001. ALM, aluminium hydroxide; Apc, adenomatosis polyposis coli; HPF, high-power field; i.n., intranasal; i.p., intraperitoneal; PBS, phosphate-buffered saline.
In analyses of epithelial EMT markers, the intensity of E-cadherin was downregulated in ApcMin/+ NP mice compared with wild type NP mice (p=0.031, figure 1F) and that of β-catenin and Sox6 was decreased in both NP mice (see online supplementary figure S1A,B). On the contrary, the expression level of the mesenchymal marker, α-SMA in the epithelial layer, was elevated in ApcMin/+ NP mice compared with the wild-type NP group (p=0.015) and ApcMin/+ control group (p<0.001), which represents the presence of EMT (figure 1G). Another mesenchymal marker, vimentin (a filament of mesenchymal cells), was overexpressed in ApcMin/+ NP mice compared with ApcMin/+ control mice (p=0.005, figure 1H). Twist (transcriptional repressor of E-cadherin gene) was also increased in both NP models (p<0.001), showing increased EMT in ApcMin/+ NP mice (figure 1I).
Supplemental material
To evaluate the molecular expression confined within polypoid lesions, we examined expression scores of EMT markers in control tissues, non-polypoid lesions and polypoid lesions in ApcMin/+ mice to verify whether EMT was more severe in polypoid lesions. Epithelial markers, including E-cadherin and β-catenin, were decreased (p<0.001) in polypoid lesions (see online supplementary figure S2A,B). Mesenchymal marker, α-SMA, was increased more in the polypoid lesion compared with control mucosa (p=0.007 and p<0.001, see online supplementary figure 2C). Consequently, these findings implied that the EMT process associated with Wnt/β-catenin signaling was activated in ApcMin/+ NP mice.
Furthermore, to confirm this phenomenon, EMT-related genes were analysed using PCR array on wild type and ApcMin/+ control mice mucosal tissues (see online supplementary figure S3). Results indicated that Snail3, Twist1, Mmp3 and Mmp9 exhibited higher expression in ApcMin/+ mice, supporting our hypothesis (see online supplementary table S5). We also analysed the histopathological characteristics that are typical of EMT (focal denudation, thickening of the mucosa and squamous metaplasia), and the ratio of EMT length was measured for the entire length of the nasal mucosa (see online supplementary figure S4A).6 Results indicate that the percentage of EMT lesions was distinctly increased in ApcMin/+ NP mice compared with the wild type NP group (p=0.014) and ApcMin/+ control group (p<0.001, see online supplementary figure S4B). Accordingly, ApcMin/+ NP mice exhibited the highest epithelial thickness (see online supplementary figure S4C).
Wnt and other signaling mechanisms in ApcMin/+ mice
Aberrantly activated Wnt signaling can promote cellular proliferation and differentiation.36 37 Therefore, we assessed both phenomena using IHC. Ki-67, a proliferation marker, was increased in both NP models and had no difference between the wild-type and ApcMin/+ NP model (see online supplementary figure S5A). Ciliated cell differentiation in the epithelial layer measured by acetyl-α-tubulin intensity was decreased in both NP models (p<0.001, see online supplementary figure S5B) and was not affected by Wnt genetic status. However, goblet cell differentiation was increased in ApcMin/+ NP mice compared with ApcMin/+ control mice (p<0.001) or wild type NP mice (p=0.022, see online supplementary figure S5C), suggesting some influence of Wnt signaling on goblet cell hyperplasia.
Since TGF-β1/p-Smad3 signaling pathway influences the activation of EMT,19 we evaluated potential involvements in this mouse model. The epithelial intensity of TGF-β1 was increased in the ApcMin/+ mice, and the intensity was higher in the NP model compared with wild-type NP mice (p=0.045). However, p-Smad3, a downstream molecule of TGF-β1, showed no differences among groups (see online supplementary figure S5D,E).
Inflammatory profiles of ApcMin/+ mice with NPs
We next examined the inflammatory burden in each mouse model using IHC staining. IL-17A was shown to be increased in ApcMin/+ NP mice compared with wild type NP (p=0.022 for septum and p=0.044 for turbinate) and ApcMin/+ control mice (p<0.001 for septum and p<0.001 for turbinate). These findings were consistent in both nasal septum and turbinate (figure 2A,B). Furthermore, the degree of neutrophilic infiltration was significantly increased in ApcMin/+ NP mice compared with the wild type NP mice (p=0.044) and ApcMin/+ control group (p<0.001, figure 2C), whereas that of eosinophilic infiltration was not significantly elevated in ApcMin/+ NP mice compared with wild type NP mice (figure 2D). The expression levels of IL-4 and IFN-γ were also increased in both NP models compared with their respective controls. However, these levels were not significantly upregulated between the wild type NP and ApcMin/+ NP mice (see online supplementary figure S6A,B). Overall, ApcMin/+ NP mice exhibited higher inflammatory profiles compared with the wild-type NP mice.
Expression of inflammatory markers in wild-type and ApcMin/+ mice. Inflammatory cells in the subepithelial layer were counted in a HPF. (A) IL-17A-positive cell counts in the septal mucosa of ApcMin/+ NP mice. (B) IL-17A in the turbinate mucosa. (C) Neutrophilic infiltration. (D) Eosinophilic infiltration in Sirius red staining. *P<0.05, **P<0.01, ***P<0.001. Apc, adenomatosis polyposis coli; HPF, high-power field; IL-17, interleukin-17; OVA, ovalbumin; PBS, phosphate-buffered saline; SEB, staphylococcal enterotoxin B; WT, wild type.
Lastly, total and OVA-specific IgE levels were increased in both NP models compared with their respective controls, and OVA-specific IgE levels were significantly upregulated in ApcMin/+ NP mice (see online supplementary figure S6C). Alternatively, expression of OVA-specific IgG1 and IgG2a were reduced considerably in the ApcMin/+ NP group compared with wild type NP mice. Spleen size was markedly enlarged in the ApcMin/+ NP mice (see online supplementary figure S6D).
Wnt signaling and EMT are inhibited by Wnt signaling inhibitor in murine NP model
BALB/c NP mice were treated with either β-catenin/CBP inhibitor, ICG-001 or dexamethasone (figure 3A). Results indicated that the number of polypoid lesions was significantly reduced to a similar degree following treatment with ICG-001 (p<0.001) or dexamethasone (p=0.006) compared with that observed in the positive control (OVA/SEB-induced NP) group (figure 3B). The nuclear β-catenin-positive cells, which represent increased Wnt signaling, were decreased after ICG-001 and dexamethasone treatment (p=0.013 and p=0.003, figure 3C). WNT3A and cyclin D1, a Wnt target molecule, were downregulated after ICG-001 and dexamethasone treatment (p<0.01, figure 3D,E).
Wnt signaling and EMT are inhibited by Wnt signaling inhibitor in murine NP model. (A) A protocol of the murine model of CRSwNP in BALB/c mice. (B) Polypoid lesions are indicated with red circles, and the number of polyp-like lesions was compared between groups. (C) The number of nuclear β-catenin-positive cells was counted in a HPF. (D) WNT3A-positive cell numbers in immunofluorescence. (E) The number of cyclin D1-positive cells per total epithelial cells. (F) The signal intensity of E-cadherin. (G) Immunohistochemical intensity of α-SMA. (H) Vimentin-positive cell counts in immunofluorescence. (I) The intensity of twist. *P<0.05, **P<0.01, ***P<0.001. ALM, aluminium hydroxide; α-SMA, α-smooth muscle actin; DEX, dexamethasone; EMT, epithelial to mesenchymal transition; HPF, high-power field; ICG-001, indocyanine green-001; i.n., intranasal; i.p., intraperitoneal; OVA, ovalbumin; PBS, phosphate-buffered saline; SEB, staphylococcal enterotoxin B.
Epithelial EMT markers, including E-cadherin and β-catenin, were also significantly elevated in both ICG-001 (p=0.003 and p<0.001) and dexamethasone-treated groups (p=0.019 and p=0.005) compared with the positive control mice (figure 3F and see online supplementary figure S7A). The expression of Sox6, another epithelial marker, was slightly increased after ICG-001 treatment without statistical significance (see online supplementary figure S7B). Alternatively, the expression levels of mesenchymal markers, such as α-SMA and vimentin in the epithelial layer, were significantly decreased in the ICG-001 therapy group compared with the positive control group (p=0.007 in figure 3G and p=0.005 in figure 3H, respectively). Twist, EMT transcriptional factor, was decreased in both treatment groups (p=0.045 and p=0.010, figure 3I).
To evaluate the process of EMT in the polypoid lesions, EMT markers were assessed in polypoid area, non-polypoid area and in control nasal mucosa. Epithelial markers, including E-cadherin and β-catenin, were decreased in the polypoid lesions compared with the control tissues and non-polypoid lesions (see online supplementary figure S8A,B), whereas mesenchymal marker, α-SMA was increased in the polypoid lesions (see online supplementary figure S8C). Expectedly, the percentage of EMT lesions and epithelial thickness was observed to be decreased in both treatment groups compared with the positive control group (see online supplementary figure S4B,D).
ICG-001 experiment showed the similar findings with ApcMin/+ mice. A proliferation marker (Ki-67) and ciliated cell marker (acetyl-α-tubulin) tended to decrease in both treatment groups without statistical significance (see online supplementary figure S9A,B). Goblet cell hyperplasia was reduced in both treatment groups compared with the positive control group (p=0.009 and p=0.018, see online supplementary figure S9C). The epithelial intensity of TGF-β1 was decreased after ICG-001 treatment, while p-Smad3 had no change after treatment, suggesting less contribution of TGF-β1 signaling on EMT in our model (see online supplementary figure S9D,E). Taken together, these findings from both animal studies might suggest that increased TGF-β1 expression may be related to crosstalk with Wnt signaling pathway.38 However, it may not be associated with EMT mechanism in this NP model since TGF-β1 signaling did not affect p-Smad3.
Inflammatory profiles after Wnt signal inhibition with ICG-001
Eosinophilic infiltration was decreased in both ICG-001 (p<0.001) and dexamethasone treatment groups (p<0.001) compared with the positive control group (figure 4A). Both ICG-001 and dexamethasone-treated groups also exhibited lower nasal mucosa mRNA levels of IL-4 (p<0.001) and IL-17 (p=0.004 and p=0.041) compared with the positive control group (figure 4B).
Inflammatory markers are underexpressed in the Wnt signal inhibition experiment. (A) Eosinophilic infiltration in Sirius red staining. (B) The mRNA expression levels of IL-4, IL-5, IFN-γ and IL-17 in the sinonasal mucosa of BALB/c mice. (C) OVA (1 mg/mL) stimulation and ICG-001 (5 µM) treatment using spleen cell culture and measuring cytokines (IL-4, IL-5, IFN-γ and IL-17) with ELISA. (D) Serum level of total IgE, OVA-specific IgE, IgG1 and IgG2a. *P<0.05, **P<0.01, ***P<0.001. DEX, dexamethasone; HPF, high-power field; ICG-001, indocyanine green-001; IFN-γ, interferon-γ; Ig, immunoglobulin; IL, interleukin; OD, optical density; OVA, ovalbumin; PBS, phosphate-buffered saline, SEB, staphylococcal enterotoxin B.
The protein levels of all cytokines (IL-4, IL-5, IL-17 and IFN-γ) in the splenic cell cultures were observed to be the highest in the positive control group following stimulation with OVA. Within the same positive control group, the expression of these cytokines was seen to be significantly suppressed following ICG-001 (p=0.044, p=0.018, p=0.044 and p=0.004, figure 4C). Additionally, serum total IgE was significantly reduced following treatment with ICG-001 (p=0.035) and dexamethasone (p<0.001), whereas levels of all other serum markers namely, OVA-specific IgE, IgG1 and IgG2a were only significantly reduced following treatment with dexamethasone compared with the positive control group (figure 4D).
Wnt signal-associated molecules are upregulated in CRSwNP tissues
Expression levels of Wnt ligands, namely, WNT1, WNT3A, WNT5B and WNT8A, were upregulated in NP tissues from CRSwNP patients (p=0.046, p=0.045, p=0.019, and p=0.033, figure 5A). Wnt receptors such as FZD1, FZD2 and FZD3 were also upregulated in NP tissues compared with all other groups, including the UP from control, the UP from CRSsNP and the UP from CRSwNP groups (figure 5B). Additionally, the β-catenin mRNA expression level was downregulated in the UP from CRSwNP group (p<0.001), and the NP from CRSwNP group (p=0.007) compared with the UP from control thereby representing the expression level present in cell-to-cell junctions, the cytosol, as well as in the nucleus (figure 5C). We, therefore, we quantified nuclear β-catenin levels, an active form of β-catenin in the epithelium, using IHC staining, and results revealed that the CRSwNP patients exhibited the highest expression in the NP tissues (p=0.042, figure 5D). WNT3A-positive cells were abundant in the UP and NP tissues of CRSwNP patients (figure 5E). FZD1-positive cells were significantly increased within the NP tissues from CRSwNP (figure 5F), whereas the abundance of FZD3-positive cells did not differ between groups (figure 5G). When we compared the mRNA expression levels of Wnt-related markers in sinonasal tissues with that of clinical parameters for CRS patients, disease severity parameters as determined by the preoperative Lund-Mackay score and Lund-Kennedy score were positively correlated with WNT10B, FZD2, and FZD3 in CRSwNP tissues, and WNT2B and WNT5B in CRSsNP tissues (table 2). Also, the preoperative symptom score, SNOT-22, was positively correlated with mRNA expression levels of WNT1. Therefore, disease extent and severity in CRS patients may be associated with the Wnt signaling pathway, which promotes tissue remodelling and EMT.
Wnt signal-related molecules are upregulated in human sinonasal tissues from patients with CRS and WNT3A-induced EMT in human nasal epithelial cells (hNECs). (A) The mRNA expression levels of Wnt ligands such as WNT1, WNT3A, WNT5B and WNT8A. (B) Wnt receptors, including FZD1, FZD2 and FZD3 were evaluated. (C) The mRNA expression of β-catenin in the sinonasal tissues. (D) Nuclear β-catenin-positive cell numbers in the sinonasal epithelium. (E–G) Positive cell counts per total cells of WNT3A, FZD1 and FZD3 in the sinonasal tissues. (H) hNECs were cultured with WNT3A (200 ng/mL) and incubated for 48 hours. The expression of Wnt signaling downstream gene (c-myc), epithelial marker (E-cadherin) and mesenchymal markers (N-cadherin and α-SMA) were detected by Western blotting. (I) Representative images of hNECs were stained with phalloidin (above), and phase contrast images (below). (J) Confocal immunofluorescence image of hNECs stained for E-cadherin, α-SMA, and DAPI. *P<0.05, **P<0.01, ***P<0.001. CRSsNP, chronic rhinosinusitis without nasal polyp; CRSwNP, chronic rhinosinusitis with nasal polyp; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; hNECs, human nasal epithelial cells; HPF, high-power field; UP, uncinate process.
Spearman’s correlation coefficients of mRNA expression levels of Wnt-associated molecules in sinonasal tissues with clinical parameters of chronic rhinosinusitis patients
WNT3A-treated hNECs released proinflammatory cytokines in a previous study,13 and our results showed increased expression of WNT3A mRNA in the tissues from CRS patients (figure 5A). To determine the effects of WNT3A on promoting EMT in hNECs, we treated WNT3A (200 ng/mL) and incubated for 48 hours. One of the Wnt/β-catenin signaling downstream gene, c-Myc was increased in WNT3A-treated hNECs. We next confirmed EMT markers, including E-cadherin, N-cadherin and α-SMA. Epithelial marker (E-cadherin) was decreased, while mesenchymal markers (N-cadherin and α-SMA) were increased in WNT3A treated hNECs (figure 5H). It also induced morphological change and increased F-actin stress fibres (figure 5I). Consistently, IF images showed the change of EMT markers (figure 5J). These results suggest that WNT3A induces EMT in hNECs.
Markers of angiogenesis (vascular endothelial growth factor and angiogenin), tissue remodelling (tissue inhibitor of metalloproteinase (TIMP)1 and TIMP2), and cyclin D1, which are known target genes for the canonical Wnt/β-catenin pathway,39 were found to be elevated in the NP tissues of CRSwNP patients compared with the UP tissues (see online supplementary figure S10A,B). TGF-β1 and p-Smad3 levels were not different between control and NP tissues (see online supplementary figure S10C,D).
Discussion
Previous studies have reported an association between NP and the Wnt signaling pathway in CRS.13 40 To the best of our knowledge, we are the first to present evidence suggesting that Wnt-induced EMT is related to the pathogenesis of NPs, using a murine model of Apc mutant mice with aberrant activation of Wnt signaling and human sinonasal tissues. Moreover, we have demonstrated functional study that inhibition of Wnt signaling with ICG-001, a selective β-catenin/CBP inhibitor, reduced NP formation and the overall inflammatory profiles as effectively as does dexamethasone treatment in a murine NP model. The IHC, IF and quantitative PCR analysis of tissues obtained from our Apc mutant NP mice as well as from CRSwNP patients, we provide evidence that the Wnt pathway may contribute to the pathogenesis of NPs, specifically through the stimulation of EMT.
Previous reports have also described that the Wnt signaling pathway is activated during inflammatory processes and tissue remodelling process specifically, within chronic lung diseases.41 42 Similar to what is observed in lower airway diseases, Wnt signaling is aberrantly activated in NPs due to epithelial damage and inflammation. A recent study on single-cell analysis of CRSwNP was shown that genes related with the Wnt signaling pathway were upregulated, and IL-4 and IL-13 exposure enhanced expression of Wnt/β-catenin.43 Böscke et al reported that human NP tissues demonstrate upregulation of Wnt signaling molecules including WNT2B, WNT3A, WNT4, WNT7A and FZD2.13 We also evaluated various Wnt signaling molecules and our results were consistent with previous studies, revealing that the level of WNT1, WNT3A, FZD1 and FZD2 were upregulated in human NP tissues.13 40 Consistently, WNT3A-induced EMT via decreasing epithelial marker and increasing mesenchymal marker in nasal epithelial cell culture. The expression levels of β-catenin, a protein with dual action in cell-to-cell adhesion and intracellular signal transduction,44 were quantified using IHC staining of non-active and active status. Nuclear β-catenin, which represents the activated form of the molecule, was upregulated in NP tissues from CRSwNP subjects (figure 5D). Meanwhile, mRNA expression of the non-active form of β-catenin in the epithelial layer was found to be the highest in the control tissues, reflecting an intact cell-to-cell adhesion.13 In our animal models, the nuclear expression of β-catenin as a surrogate for Wnt/β-catenin activity was increased in the ApcMin/+ NP mice and decreased after ICG-001 treatment. However, the junctional expression of β-catenin for the epithelial marker of EMT was the opposite of the nuclear β-catenin.
Canonical Wnt/β-catenin signaling has been a primary focus for disease pathogenesis, especially in lung diseases, including idiopathic pulmonary fibrosis, pulmonary hypertension, COPD and asthma.18 It has been demonstrated in an animal study that active β-catenin promotes ectopic differentiation of alveolar cells, goblet cell hyperplasia and tissue remodelling.37 Similar to these previous studies, our study also revealed that aberrant Wnt/β-catenin signaling in ApcMin/+ mice affected nasal polypogenesis leading to tissue remodelling and EMT as well as increased airway inflammation.
Increased EMT was observed in both murine NP models (ApcMin/+ NP mice and BALB/c NP mice), which were confirmed by examining epithelial changes and EMT markers. Most nuclear β-catenin and the mesenchymal marker (α-SMA) were significantly upregulated in ApcMin/+ NP mice. Neutrophilic inflammation was enhanced in ApcMin/+ NP mice. The number of cells expressing IL-17A was also increased, whereas those expressing IL-4, as well as eosinophilic infiltration, were not significantly affected. Cellular markers of proliferation (Ki-67), cilia (acetyl-α-tubulin) and goblet cells (PAS staining) were examined. The number of goblet cells was increased in the ApcMin/+ mice compared with the wild type mice, and that was reduced following ICG-001 treatment. However, the proliferation and ciliated cell markers were not different between wild type and ApcMin/+ mice, and ICG-001 treatment showed no significant changes. Collectively, it was thought that activation of β-catenin itself as well as aberrant mucosal inflammation may induce goblet cell hyperplasia.37
Overexpression of other EMT-related markers (vimentin, twist and cyclin D1) was observed in the ApcMin/+ mice. ICG-001 disrupts the interaction of CBP and β-catenin and antagonises β-catenin/T cell factor-mediated transcription of Wnt target genes.25 The therapeutic effects of ICG-001 have been reported in various diseases, such as liver fibrosis, myocardial injury and glioma.45–47 In this NP model, ICG-001 treatment reduced EMT, and overall inflammation of sinonasal mucosa was reduced accordingly.
This NP mouse model was initially established using BALB/c mice susceptible to Th2 inflammation as previously described.48 Intranasal instillation of OVA and SEB after OVA sensitisation induces both eosinophilic and neutrophilic inflammation which shows a similar molecular pattern and cytokine expression to the NP tissues from CRSwNP patients. The mouse NP model showed increased molecular expression of IL-4, IFN-γ, IL-17A, TGF-β1 and IL-25, and these inflammatory molecular drivers were identical to those of CRSwNP patients’ tissues.23 31 and Th1 and Th2 inflammatory markers were elevated in the NP model and EMT was also similar to human NP tissues. The therapeutic effects were studied using various anti-polyp agents, including dexamethasone as conventional treatment of NPs.6 23 24 31 Also, C57BL/6J mice exhibited polypoid changed of sinonasal mucosa and similar inflammatory patterns with BALB/c mice.24 Polypoid lesions in mice are defined as elevated mucosa with infiltration of inflammatory cells. They are less prominent than human NPs and are similar to the early stage of polypoid change in the sinonasal mucosa in patients with CRSwNP. Comparing NP model in both mouse strains, sites and shape of polypoid lesions were the same, while the number and phenotype of polypoid lesions in the wild type of C57BL/6J mice were slightly lower and smaller than that of BALB/c mice.
TGF-β1 is known to upregulate in tissues from CRSsNP patients.49 Stimulation with TGF-β1 can induce Wnt/β-catenin pathway in pulmonary fibroblasts inpatients with COPD.50 Both upper and lower airway diseases have similar histopathologic features such as fibrosis, tissue remodelling, and extracellular matrix production, and those are associated with TGF-β1 expression in the airway tissue.51 We checked the expression level of TGF-β1 and p-Smad3 in the sinonasal mucosal tissues from human and mice. Although there was no difference between control and NP tissues in human, ApcMin/+ mice exhibited upregulation of TGF-β1. EMT accompanied by β-catenin activation would have been somewhat affected by TGF-β1 signaling, but the influence of β-catenin activity itself is greater in EMT. IL-17A induced EMT has been reported in several studies and suggested mechanisms vary including canonical NF-κB/ZEB1 signaling pathway, TGF-β1-Smad-ERK signaling pathway and AKT pathway.52–54 A recent study demonstrated that the noncanonical Wnt pathway induced IL-17A secretion in mice treated with intranasal Streptococcus pneumoniae vaccine.55 In the present study, the Wnt pathway is associated with IL-17A and neutrophilic inflammation. The Endotype of CRSwNP is divided into eosinophilic and non-eosinophilic polyps and main inflammatory players of the latter Th1/Th17 inflammation.56 57 Wang et al reported that Th2 and Th17 inflammatory pathways regulate each other, and Th17 cytokines enhance Th2 pathways.58 In this study, ApcMin/+ NP mice exhibited neutrophilic and Th17-prone inflammatory features. Thus, further research is needed to identify the signaling pathway of Wnt activation via Th17 or Th1 inflammation in nasal polypogenesis.
Emerging evidence has been published suggesting that EMT, which can occur during tissue remodelling and fibrosis, is associated with CRS pathogenesis and that EMT is correlated with the clinical severity of CRS as determined through CT scores.7 24 One of the critical pathways that promote EMT is the canonical Wnt/β-catenin pathway which has been mainly studied in the context of cancer progression and metastasis.14–16 Research in lower airway diseases has also revealed that an aberrantly activated Wnt pathway exaggerates EMT, which regulates tissue repair via extracellular matrix deposition, fibroblast proliferation, myofibroblast differentiation and airway small muscle cell proliferation.36 Consistent with previous studies on inflammatory lung diseases, our data suggest that the Wnt/β-catenin pathway-induced EMT plays a crucial role in tissue remodelling and polypogenesis of CRS. Furthermore, these pathologic processes were effectively inhibited by ICG-001, an inhibitor of β-catenin/CBP signaling pathway, similar to treatment with dexamethasone in a well-established NP murine model.
Strengths and limitations
Our study is successfully established murine NP model with ApcMin/+ NP mice and defined the role of Wnt-induced EMT in the pathogenesis of NPs. The hypothesis has also been proved by functional inhibition study with an inhibitor of the β-catenin/CBP signaling pathway on the murine NP model. Wnt signaling inhibitors, such as ICG-001 may be potential targets for humans for the therapeutic purposes of CRSwNP, and the effectiveness of ICG-001 treatment in our experiment was as potent as dexamethasone treatment.
The shortcoming of this study is that we could not analyse each Wnt signaling pathway, specifically. Overall expression levels of nuclear β-catenin were significantly elevated, which reflecting activation of Wnt signaling. However, no particular Wnt signal was identified as well as the function of the non-canonical Wnt pathway was not evaluated in CRSwNP. The further molecular investigation is necessary to elucidate the pathological association between Wnt signal and NPs.
Conclusion
Our data indicate that Wnt-induced EMT is associated with the pathogenesis of NPs in a murine model using Apc mutant mice and human sinonasal tissues from CRSwNP patients. Additionally, we suggest that inhibition of Wnt signaling with ICG-001 may be effective as a potential therapeutic target to reduce NP formation and the inflammatory burden of CRSwNP patients.
References
Footnotes
H-WS and J-HM are joint senior authors.
J-SB and GR contributed equally.
Contributors Conception and design: J-SB, GR and J-HM; acquisition of data: J-SB, JHK, EHK, YHR and J-HM; analysis and interpretation of data: J-SB, GR, JHK, Y-JC, DWK, ShL, H-WS and J-HM; drafting the article or revising it critically for important intellectual content: J-SB, GR, DWK, P-SC, H-WS and J-HM.
Funding This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2016R1A2B4010407 and NRF-2020R1A2C1012105) and by the Ministry of Education (NRF-2020R1A6A1A03043283) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by Ministry of Health & Welfare, Republic of Korea (grant number: HI15C1524 and HI17C1669).
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval The study was approved by the Institutional Review Board of the Dankook University Hospital (IRB No. 2012-11-008-002).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request to the corresponding authors.