Article Text
Abstract
Digital healthcare is a rapidly growing healthcare sector. Its importance has been recognised at both national and international level, with the WHO recently publishing its first global strategy for digital health. The use of digital technology within cystic fibrosis (CF) has also increased. CF is a chronic, life-limiting condition, in which the treatment burden is high and treatment regimens are not static. Digital technologies present an opportunity to support the lives of people with CF. We included 59 articles and protocols in this state-of-the-art review, relating to 48 studies from 1999 until 2019. This provides a comprehensive overview of the expansion and evolution of the use of digital technology. Technology has been used with the aim of increasing accessibility to healthcare, earlier detection of pulmonary exacerbations and objective electronic adherence monitoring. It may also be used to promote adherence and self-management through education, treatment management Apps and social media.
- cystic fibrosis
- psychology
- exercise
Statistics from Altmetric.com
Introduction
The digital technology sector is a rapidly growing industry, with recent estimates suggesting digital technology is worth £184 billion to the UK economy1 and US$1351 billion to the US economy.2 Digital technology has become more accessible than ever before, especially among children and young people, with the Royal College of Paediatrics and Child Health describing this generation as ‘digital natives’; growing up surrounded by digital information. It is also an emerging field within the healthcare sector. It is widely used among the general population to track and promote health changing behaviours with devices such as exercise trackers and fitness Apps. Disease-specific interventions are also emerging. In COPD and asthma, there has been a move towards the use of digital technologies in the ongoing monitoring of disease and adherence promotion. Strategies include text messaging reminders and web-based and mobile applications to monitor and record symptoms.3 4 Its importance has been recognised by the WHO who recently published its first global strategy for digital health. This brought together evidence for digital health interventions currently in use and provided recommendations for future development.5
Cystic fibrosis (CF) has also seen the application of digital technologies. CF is an autosomal recessive, multisystem disorder. In the UK, there are around 10 000 people living with the condition, of which 40% are under the age of 16 years.6 In the USA around 34 000 people have CF,7 with the predicted life expectancy of 46 years for those born in 2017.8 CF is caused by abnormal functioning of the cystic fibrosis transmembrane conductance regulator (CFTR), responsible for the transport of chloride and regulating the movement of water and ions across epithelial surfaces. Based on the CF pig model, a defect in the CFTR protein may lead to abnormal mucous pH in the periciliary fluid and mucous stasis which is primarily responsible for the multisystem manifestations of CF.9 10 Specialist CF centres in conjunction with developments in treatments, improvements in antibiotic therapy and better nutrition have improved outcomes for people with CF (pwCF).11 However, treatment regimens have become increasingly complex, with many patients prescribed daily airway clearance techniques (ACTs), exercise, inhaled and nebulised medications, pancreatic enzyme replacement and dietary supplementation. Intravenous antibiotics are required for acute pulmonary infections, often more frequently as patients get older.
Despite the complexity of CF therapies, much of the regimen can be completed in the patient’s home, allowing the integration of treatments into everyday routines, with regular monitoring from the CF team. However, treatments are tiring, time-consuming and burdensome; the average time spent on treatments for children in the UK is 137 min/day and 150 min in adults.12 In addition, the routines of adults and families of children with CF are not static; managing the condition is a dynamic process involving ongoing adaptation and readjustment.13 Managing treatments alongside daily life activities can therefore be challenging and restrictive. Digital technologies present an opportunity to support and improve the lives of pwCF. Indeed, pwCF and parents acknowledge the role of technology and there are a range of applications and platforms that have been designed by them for pwCF.
The aim of this state-of-the-art review is to provide comprehensive overview of the evolution of CF-specific digital technologies and their effectiveness in the promotion of home monitoring, adherence or self-management in what is a rapidly changing area of medicine.
Methods
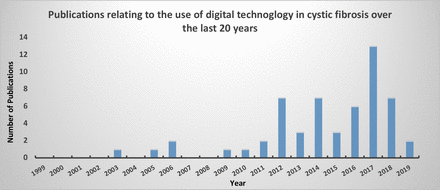
We conducted a systematic literature search of electronic databases and clinical trials registers from 1 January 1999 to 14 February 2019. The protocol with full search strategies and inclusion criteria can be found at https://nottingham-repository.worktribe.com/output/2044553. Broad search criteria were used and full text, article abstracts, conference abstracts and trial protocols were considered for inclusion in order to increase sensitivity. Once duplicates were removed, a total of 1968 electronic search results were identified which were imported into Covidence14 and considered for inclusion. The search results were reviewed independently by two reviewers. We excluded 1665 articles on title alone, leaving 303 for full text screening, of which 51 articles met criteria for inclusion. Searches of clinical trials registered over the same time period identified 48 protocols, which following duplicate removal and screening, identified a further 8 studies. Once the final articles had been identified, data were independently extracted by both reviewers with results collated into MS Excel. We included 59 articles and protocols (online supplementary table 1), relating to 48 studies. The general quality of the evidence was low; mainly consisting of small interventional and before after studies with only three full text randomised-control trials (RCTs) and one systematic review. Figure 1 highlights the expansion of technology over the last 20 years. Although for the purposes of this review technology has been assessed for its role within either home monitoring, measuring adherence or self-management we recognise that in practice these are often inter-related concepts in relation to CF.
Supplemental material
Publications relating to the use of digital technology in cystic fibrosis over the last 20 years.
Digital technology used for home monitoring
Increasing accessibility to healthcare
Centralisation of care means that many patients do not live in close proximity to their CF centre which potentially becomes a barrier to accessing healthcare. Therefore, digital technology has been explored as an option for remote delivery of care. Videoconferencing has been used for delivery of routine appointments, annual assessments and multidisciplinary team (MDT) discussions,15 with more recent studies also able to share imaging, educational slides, lung function and microbiology results. Although there was no significant difference in FEV1 results reported with teleconferencing, one study described positive patient satisfaction and 63% felt it was as good as a face-to-face review.16 It has also been used more widely for delivering mental health services to pwCF online.17 These studies again were small interventional studies and larger RCTs are required to fully assess the impact on telehealthcare in remote delivery of care. An ongoing RCT, VIRTUAL-CF is using videoconferencing to the CF MDT with remote spirometry and oxygen saturation measurements for patients receiving intravenous antibiotics in the community. It is assessing whether this approach promotes health-related quality of life compared with standard community intravenous antibiotics care.18
Home monitoring for early detection of pulmonary exacerbations
Despite life expectancy improving, the majority of deaths in CF are still attributable to respiratory failure secondary to recurrent pulmonary exacerbations. Exacerbations also contribute to morbidity; worsening CF-related diabetes and reducing health-related quality of life.19–21 Therefore, the use of digital technology has been evaluated to determine if it can lead to the earlier detection of exacerbations and subsequently reduce the rate of respiratory decline. One of the first uses of digital technology in this context was in the 1980s for home monitoring of respiratory symptoms and lung function.22 This, along with other earlier studies, used a combination of home spirometry, measuring physical parameters such as heart rate and oxygen saturations, blood glucose levels and respiratory symptom scoring with the results collated and sent via modem internet to the CF centre.23 In more recent work, advances in technology have allowed streamlining of data transmission via Wifi or Bluetooth. A small non-randomised interventional study showed promising results in FEV1 status. Data collected from a Spirotel device (Medical International Research, Rome, Italy) were sent via email to the CF centre, with patients contacted if they met intervention criteria for an exacerbation based on FEV1 decline or oxygen saturations.24 A significantly smaller decline in annual FEV1 status was noted in the telehealth group over a 4.5-year period. However, these results were not subsequently supported by a large multicentre RCT, the early intervention in cystic fibrosis exacerbation (eICE) trial.25 26 Using twice weekly home spirometry (Viasys AM2 device, CareFusion, California, USA) and respiratory symptom scoring, patients were contacted by the CF team if a reduction in FEV1 >10% or an increase in respiratory symptoms was seen. The early intervention group had a shorter time to first exacerbation and more exacerbation treatments compared with the control group; however, this was not associated with a slower FEV1 decline. The trial was stopped early for futility, as completing the trial was unlikely to show a difference in primary end point.27
However, the eICE study highlights the issue of adherence. In a condition where treatment burden is already high, home monitoring adds an additional task for the patient to complete. Home monitoring studies have required data entry from once a month to three times a week.28 29 Adherence to telemonitoring in the eICE trial over 52 weeks was suboptimal; 50% of patients transmitted data once per week and only 19% twice per week as per the protocol, with an increased treatment burden score in the early intervention telemonitoring group.26
Ongoing studies
Current studies are assessing the use of smartphone applications for the earlier recognition of exacerbations through changes in respiratory symptoms. They hope to establish whether having monitoring which is less restrictive and more mobile would improve adherence and therefore outcomes.30 31 Unfortunately, preliminary data from RCT (ACTRN12615000599572) suggest that the use of a smartphone for symptom reporting had no effect on the number of courses of antibiotics or days of antibiotic treatment, with full results awaited.32
Digital technology for monitoring and promoting adherence and exercise
Home monitoring for supporting adherence
Adherence monitoring enables treatment guidance and allows clinicians to differentiate if changes in a patient’s condition are related to disease progression, attributable to poor adherence or a combination of the two. The impact of home monitoring on treatment adherence in adolescents and young adults has been explored in a before and after study using once weekly home monitoring and adherence monitoring using medication prescription refill data to calculate the medication possession ratio (MPR).33 In comparison to Lechtzin’s eICE study, adherence to weekly spirometry monitoring was 59% which may reflect the impact of parental supervision in this population group. Less frequent monitoring may have lessened the treatment burden to patients. There was a small associated overall increase in medication adherence; MPR was 60% in the year prior to study enrolment, and 65% during the 12-month study period (p=0.038). These authors found no change in the number of exacerbations or FEV1 decline between groups across the study period.33
Digital technologies for adherence
Self-monitoring also helps patients better understand and self-manage their condition. However, self-reporting of adherence is notoriously inaccurate and so alternative ways for adherence monitoring are being pursued. Prescription refill data and MPR are a valid and inexpensive way of monitoring adherence; however, this is still prone to inaccuracies, as it is based on the assumption that all of the medications refilled are always taken.33 Hence, digital technologies are being developed to more accurately record treatment adherence.34
One small intervention study compared self versus digital electronic adherence recording of a high-frequency chest wall compression vest (Vest System, Hill-Rom, Indiana, USA). Although the results showed a high variability in self-reporting, it supported the view that patients over-estimate treatment duration; by 127% in adults and 26% in parents of children wCF over a 2-week period. Overall average treatment adherence was 69%.35
An established digital technology for adherence monitoring in CF is the data logging nebuliser device which combines routine nebuliser treatment with accurate adherence logging, via an electronic data capture facility. This gives an insight into how often and how long treatments are taking to build a picture of adherence. One such device is the I-Neb (Respironics, Chichester, UK). It is an adaptive aerosol delivery system which adapts medication delivery to the patient’s breathing pattern, delivering only during inspiration.36 37 It also provides patients with visual and audio feedback while they undertake their treatments. Its data capturing facility allows trends to be identified. For example: although one study found adherence was maintained between 60% and 70% over 1 year, diurnal variation was also noted; evening 70% versus 58% morning (p=0.012).36 This allows for more realistic joint goal setting and changes to treatments based on the results.35 36 However, this device is currently only available to a subset of patients prescribed Promixin (colistimethate sodium, Profile Therapeutics plc, West Sussex, UK) and although data logging devices may provide useful information to the CF team regarding adherence, some patients may find this level of monitoring intrusive.
Insight Online (Respironics, Chichester, UK) has been developed to be used alongside the I-Neb. It is a home monitoring telehealth interface, where patients upload data from the I-Neb to a server. The data are then analysed and presented for patients and clinicians to view. Patients are encouraged to set treatment targets, against which they can self-monitor their progress. Data presented from a small intervention study had mixed results. This showed that for the patients who did engage it improved adherence; however, over 50% of the participants failed to upload regularly.38 This result again raises the issue of increased treatment burden on patients, and that CF teams’ perceived benefit of technology may not be shared by all patients.
Digital technologies for exercise
The video gaming industry is a continuously growing global industry accessed by 64% of the US population. Of those using game consoles, 76% played for at least 3 hours a week with the majority using it for significantly longer.39 Exercise is a key treatment in CF and, given the popularity of gaming technology, incorporating exercise through gaming has been explored as a strategy to help people to engage and self-monitor exercise. There have been small interventional studies using games consoles for promotion of home-based exercise.40 An RCT used the Nintendo Wii EA Sports Active 2 to deliver a 6-week training programme of 30–60 min sessions, 5 days a week, monitored by a virtual personal trainer, with participants followed up for 12 months following the intervention.41 42 Unfortunately, this did not support the use of video gaming. Although both control and intervention groups showed a significant difference between physical parameters prestudy and poststudy, there was no difference between groups. In addition, although short-term adherence was good, with 95% adherent at 6 weeks, this was not sustained and reduced to 35% using the game twice a week by 12 months, with 65% not using it at all.41 A potential reason is that if games are played frequently over an extended time period there is an element of monotony, similar to treatment regimens.
Fitness trackers have been used within the general population for some time to promote exercise, with CF-specific trials focused mainly from 2017 onwards. An RCT by Bishay compared a fitness tracker with a personalised exercise prescription and social media platform to exercise prescription alone over a 12-month period.43 This study did not support the use of fitness trackers in CF with no significant difference between the two groups in terms of exercise tolerance, pulmonary function or patient-reported outcomes.
There are other examples of social media and web-based platforms for exercise promotion. A small interventional pilot study used a closed Facebook group to promote a 30-day exercise challenge to increase daily exercise.44 Pactster in the UK (developed with the CF Trust) provided online exercise classes and included instructors who have CF.45 ‘CFYOGI’ in the USA46 is an exercise web platform cofounded by a pwCF and a parent of children with CF in partnership with Social Good Fund. It includes livestreamed and recorded fitness videos led by instructors with CF and has community features allowing patients to share progress and fitness goals while avoiding cross-infection risks.
Ongoing studies
The development of data-tracking nebulisers has led to the development of CFHealthHub observatory,47 a large scale data observatory which aims to recruit 6000 pwCF over the period 2017–2021 and collect observational data using patients’ data tracking nebulisers. In addition, it will be used as part of a large multicentre RCT (ISRCTN55504164)48 which will combine the use of data captured from data tracking nebulisers with behavioural change interventions available via a web portal, CFHealthHub. Data are available to be viewed by both clinicians and pwCF, with the primary outcome of the study being number of exacerbations. Second, a non-randomised crossover study (NCT02700243) is exploring the use of video games in improving adherence to ACTs using a positive expiratory pressure (PEP) device. Electronic versus self-reporting of adherence will be monitored over a 4-month period, following which a video game will be integrated into the PEP device operated by the patient performing their therapy correctly. Primary outcome is adherence to therapy, with change to pulmonary function a secondary outcome.49
There are several ongoing studies in relation to exercise.50 ACTIVATE-CF,51 is using pedometers and daily web-based logging of exercise activities in addition to the standard care of 3 monthly counselling sessions to promote physical activity. Patients are encouraged to exercise 3 hours a week, with FEV1 status being the primary outcome. Another RCT (NCT03672058) combines the use of Fitbit exercise trackers (Fitbit, California, USA) with an online activity monitoring system (Fitabase) and personalised feedback on activity levels and progress, compared with the use of a Fitbit alone on steps per day and FEV1 status.52 Finally, project Fizzyo combines their use with ACT devices to assess activity levels, with chipped ACT devices used to capture daily adherence data.53 Data transmission appears more streamlined compared with other studies, with the results sent automatically once the ACT device is synchronised with a tablet computer. In addition, computer games have been developed in conjunction with patients to be used at different intervals within the study to assess the impact of gaming on adherence.53
Digital technology for education and self-management
Self-management is key to successful management of chronic conditions such as CF. Self-management has been described as the process of helping patients and their families to choose, monitor and adjust their treatment requirements in relation to their condition and the effect it has on their lives.54
Education
Multiple digital technology platforms have been explored to promote self-management through education delivered as either self-guided sessions or mentor-supported individual or group web-based sessions. While they may provide educational opportunities for participants, the self-management education and digital technologies themselves have had limited success, so far, in relation to patient outcomes. This is in keeping with a Cochrane review that suggested self-management education in CF could provide limited positive change for a small number of self-management behaviours.54
Early education strategies explored education delivery via a CD-Rom, combining a computer game with education.55 Several platforms have since been developed. Web-based education has increased in popularity, and the use of smartphones and Apps has been developed.56 CFfone provided access to the CFfone website via a smartphone, which contained educational materials and social support through inspirational stories and communication with peers in an online community.57 The BeInCharge study for parents of children with CF combined an education programme on dietary education, personalised calorie intake goals and behaviour techniques with a daily diet tracker App. Use of the App resulted in no significant increase in weight gain in the intervention group and indeed the App was less successful in achieving weight gain compared with the face-to-face delivery used previously.58
Individual education delivery for both children and adults guided by a mentor or physio has been explored. Patients in a small RCT, with two intervention treatment arms, used an online mentor-guided self-efficacy programme with or without an App to monitor their symptoms and quality of life, compared with the control group.59 Results were limited by the small sample size, although patients reported being confident in using digital technology. An increase in self-efficacy was seen across the intervention groups; however, this was similar in both groups with and without the use of the App. Similarly, group sessions in CF have been limited to small interventional trials.60 Results of larger studies are required, one of which has recently been completed.61 This delivered six online modules relating to nutrition, medications and respiratory and liver disease. Patients also completed tasks and had access to videos of patients sharing their experiences. Preliminary data at 6 months show mixed results, with adherence data awaited. Significant improvements in body mass index and ADE vitamin levels were seen, however this did not translate to a reduction in lung function decline.61 62
Ongoing studies using education
Two large RCTs addressing this issue are due to be completed in 2019. Project UPLIFT provides group web-based education and intervention programme focusing on improving anxiety and depression in pwCF.63 Adherence is a secondary outcome. It focuses on education on depression and CF; coping strategies such as cognitive behavioural therapy and mindfulness; and relaxation techniques. An Australian project CyFiT,64 encompasses several aspects of digital healthcare delivery. It combines the delivery of outpatient physiotherapy via videoconferencing, with additional multimedia and educational features. Participants are also given an activity tracker to record sleep and physiological parameters to guide exercise prescriptions and the telehealth sessions. The primary outcome is pulmonary function and the comparator is standard physiotherapy. Finally, a pilot study for an RCT (NCT03637504) plans to evaluate the feasibility of MedActionPlan (MedActionPlan, Peapack, USA); a web-based medication management App which uses education about treatment regimens to encourage self-management and adherence. Adherence is assessed using eTrack nebulisers and AdhereTech pill bottles (AdhereTech, New York, USA).65
Digital technology for self-management of treatments
Digital Apps are being used in a variety of contexts within CF including symptom monitoring, education and diet tracking. A newer area of interest is the development CF-specific Apps to help manage treatment regimens. ‘Genia’ is a Swedish-based treatment management App developed by a father and his daughter, who has CF,65 which allows pwCF to track their condition as well as providing the option to share information with care teams about symptoms, daily activities and medications, as well as sharing updates with family and friends.
Ongoing studies
In the context of pancreatic insufficiency, smartphone Apps are under development which will be capable of calculating patient-specific PERT requirements.66–68 MyCyFAPP is a multicentre, multidisciplinary development currently being tested which aims to allow patients to better self-manage their PERT and nutrition. Importantly, parents and patients of various ages were involved in the development phase as part of interviews and focus groups. The protocol describes a number of expected features of the App including education through games, food and symptom scoring records and a handbook containing nutritional information, in addition to calculating optimal PERT dosing. These data are also accessible to clinicians with alerts for suboptimal treatment, with the aim of promoting more focused and realistic goal setting at follow ups.68
The use of social media for self-management in CF
Social support is known to improve self-management and therefore the use of social media to provide social support has been explored, although CF-specific research is limited. A thematic analysis undertaken on a CF charity website message board found that online support groups seem to supplement professional support in relation to self-management and self-esteem.69 Social media has also been incorporated into projects such as CFfone (described previously). No larger CF-specific studies were identified.
Conclusion
Digital technology is a growing industry. We have highlighted the expansion and evolution of its use in CF over the last 20 years for supporting home monitoring, adherence and self-management. Despite the large number of articles, most were small pilot and intervention studies without comparators and there were very few full text RCTs. Although ongoing studies may yield some positive results, the majority so far have shown limited evidence to support the use of digital technology. An area of promise was electronic monitoring of adherence via data logging nebulisers to accurately capture adherence data. However, this does not address barriers to adherence. In addition, a potentially exciting area of development is the use of digital technology to assist in the self-management of medications, such as Apps providing patient-specific PERT dosing information. Future evaluation of the role of digital technology in CF will require well designed, adequately powered RCTs. Developers should be mindful that, in a condition where there is already a significant treatment burden, patients must find these technologies acceptable and sustainable. The benefits of digital technology must be carefully balanced against the investment of time needed to use them. Patient involvement in the design process is key. Furthermore, the benefits of treatment should be of benefit to the patient as well as the CF team.
References
Footnotes
Twitter @DrRCalthorpe
Contributors RJC and SS completed the systematic literature search and data extraction. KC wrote the review with all authors commenting on the final manuscript. In addition, KG also contributed to the review by the inclusion of the patients perspective throughout the article. ARS was the supervising author on this review, and is the corresponding author.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests ARS has provided consultancy for Vertex and holds a current unrestricted research grant from Vertex. He has taken part in clinical trials sponsored by Vertex, Raptor and Insmed. He has given lectures at meetings sponsored by Teva and Vertex.
Patient consent for publication Not required.
Provenance and peer review Commissioned; externally peer reviewed.