Article Text
Abstract
Background Most patients with amyotrophic lateral sclerosis (ALS) are treated with mechanical insufflation–exsufflation (MI-E) in order to improve cough. This method often fails in ALS with bulbar involvement, allegedly due to upper-airway malfunction. We have studied this phenomenon in detail with laryngoscopy to unravel information that could lead to better treatment.
Methods We conducted a cross-sectional study of 20 patients with ALS and 20 healthy age-matched and sex-matched volunteers. We used video-recorded flexible transnasal fibre-optic laryngoscopy during MI-E undertaken according to a standardised protocol, applying pressures of ±20 to ±50 cm H2O. Laryngeal movements were assessed from video files. ALS type and characteristics of upper and lower motor neuron symptoms were determined.
Results At the supraglottic level, all patients with ALS and bulbar symptoms (n=14) adducted their laryngeal structures during insufflation. At the glottic level, initial abduction followed by subsequent adduction was observed in all patients with ALS during insufflation and exsufflation. Hypopharyngeal constriction during exsufflation was observed in all subjects, most prominently in patients with ALS and bulbar symptoms. Healthy subjects and patients with ALS and no bulbar symptoms (n=6) coordinated their cough well during MI-E.
Conclusions Laryngoscopy during ongoing MI-E in patients with ALS and bulbar symptoms revealed laryngeal adduction especially during insufflation but also during exsufflation, thereby severely compromising the size of the laryngeal inlet in some patients. Individually customised settings can prevent this and thereby improve and extend the use of non-invasive MI-E.
- Non invasive ventilation
- Cough/Mechanisms/Pharmacology
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
What is the key question?
Mechanical insufflation–exsufflation (MI-E) is an efficient tool used to improve cough in most patients with neuromuscular disorders, but the method often fails when bulbar involvement is present.
What is the bottom line?
We used laryngoscopy during ongoing MI-E and saw that patients with bulbar amyotrophic lateral sclerosis (ALS) were prone to adduct laryngeal structures throughout the various pressure cycles, thereby severely obstructing the airflow and the effect of the treatment.
Why read on?
In patients with bulbar ALS, cough assistance with MI-E should be delivered carefully and according to the criteria suggested in the present study.
Introduction
Amyotrophic lateral sclerosis (ALS) is an incurable and highly disabling neurodegenerative disease of upper and lower motor neurons. Treatment is largely symptomatic, and average life expectancy at the time of the diagnosis is 2–3 years unless ventilatory assistance is provided.1
ALS is classified as ‘spinal’ if symptom onset affects the limbs predominantly, and as ‘bulbar’ if the disease presents with difficulty in speaking, swallowing or coughing. Paresis to predominantly upper motor neurons leads primarily to spasticity, whereas paresis of lower motor neurons leads to flaccidity.2 Regardless of the subtype, ALS progresses and eventually encompasses all skeletal muscles.3 Involvement of respiratory muscles limits respiratory function and cough, thereby leading to secretion accumulation, lung infections and, eventually, respiratory failure.3–6 Effective augmentation of cough is vital for clearance of airway secretions in these patients and fundamental for the prevention and treatment of pneumonias.6 ,7
In a voluntary cough, inspiratory muscles increase the lung volume, laryngeal muscles coordinate opening and closure of the glottis and expiratory muscles increase the thoracoabdominal pressure.8 These interactions are disturbed in neuromuscular disorders.7 ,9 Mechanical insufflation-exsufflation (MI-E) is used widely to assist cough mechanically by applying positive and negative pressure changes to the airways, either non-invasively via a mask or invasively via a tracheostomy.10 ,11 It has been hypothesised that coordinated glottic movements are required for MI-E to be effective.12 Non-invasive MI-E can be difficult to apply in patients with the bulbar subtype of ALS. This problem may be due to dysfunction of bulbar-innervated muscles, but the basic mechanisms are not understood.
The laryngeal response to MI-E in patients with ALS has never been studied. Here, we investigated the laryngeal response patterns to MI-E in ALS to improve the treatment that we can offer to these severely ill patients.
Methods
Neurological assessment and definitions
ALS was diagnosed by a senior neurologist (O-BT) in accordance with the revised criteria set by the El Escorial World Federation of Neurology.13 ,14 The disease was classified as ‘spinal ALS’, ‘ALS with progressive bulbar palsy’ (hypotonic bulbar onset with dysarthria, tongue atrophy and absence of jaw reflex) or ‘ALS with pseudobulbar palsy’ (spastic bulbar onset with dysarthria, exaggerated jaw reflex and no tongue atrophy). Patients were assessed using the ALS Functional Rating Scale-revised (ALSFRS-r).15 Bulbar impairment score (BIS) was evaluated from the ALSFRS-r, from where the items of speech and swallowing were calculated.16 Dysphagia was determined using the 100 mL water swallow test.17 ,18
Subjects
This was a cross-sectional observational population-based study of 20 patients with ALS who had not undergone tracheostomy and 20 neurologically healthy age-matched and sex-matched controls. Exclusion criteria were age <18 years, history of laryngospasm, sensitisation to Xylocain (anaesthetic used during laryngoscopy), pneumothorax, additional lung disease, cancer, acute infection of the chest 1 month before study commencement and mental instability.
Approximately 20 patients with ALS who have not undergone tracheostomy are usually enrolled at all times at the ALS clinic at Haukeland University Hospital (Bergen, Norway), which serves a population of ≈500 000 inhabitants. At the start of this study, 17 patients were enrolled at the clinic and 20 new patients were diagnosed and enrolled during the 1.5-year recruitment period from December 2011 to June 2013. All 37 patients were informed about the study and invited to participate. Thirteen patients declined and four died soon after being invited, leaving 20 participants. Reasons for non-participation were severe disease/fatigue (n=7), or limb-onset ALS without bulbar symptoms and, therefore, no interest in participation (n=6). The study protocol was approved by the Regional Committee for Medical Research Ethics. Written informed consent was obtained from all participants.
Pulmonary function and respiratory strength
Spirometry was undertaken with a Vmax 22 Encore system (SensorMedics, Yorba Linda, California, USA). FVC, FEV1 and peak expiratory flow were measured seated, with a nose clip. Slow vital capacity was measured with a Respirometer (nSpire Health, Hertford, UK). Peak cough flow was measured using a hand-held Peak Flow Meter (Vitalograph, Ennis, Ireland). Plateau values (average of 1 s) of the maximal inspiratory (Pimax) and expiratory (Pemax) muscle strength and sniff nasal inspiratory pressure (SNIP) were measured seated using a Respiratory Pressure Meter (Micro RPM; Micro Medical, Rochester, UK). SNIP was measured at functional residual capacity, Pimax at residual volume and Pemax at total lung capacity. The highest value from three or more attempts was selected for analyses and standardised to predicted percentages.19–22
Video-recorded transnasal fibre-optic laryngoscopy during MI-E
Video-recorded transnasal fibre-optic laryngoscopy (ENF-P3; Olympus, Tokyo, Japan) was used to visualise laryngeal anatomy at baseline and response patterns during MI-E (Cough Assist; Respironics, Murrysville, Pennsylvania, USA). We used a set-up described in detail previously, except that the laryngoscope was supported manually (see online supplementary figure S1) instead of using a customised headgear.23 A standardised MI-E protocol was used.23 The protocol comprised 12 intervention arms with various combinations of pressures, instructions and manual thoracic thrust (see online supplementary table S2).
Supplemental material
Pressures of ±20, ±30, ±40 and ±50 cm H2O were used with specific instructions. For MI-E in automated mode with 2 s insufflation, 2 s exsufflation and 1 s pause, the instructions were to ‘inhale’ actively when insufflation was started and to (A) ‘exhale’ or (B) ‘cough’ actively when the device switched to exsufflation. For MI in manual mode with 2 s insufflation followed by manually assisted thoracic thrust, the instructions were to ‘inhale’ actively when insufflation was started and to ‘cough’ actively during the thoracic thrust.
In case of patient discomfort, the procedure was stopped and higher examination pressures were not applied.
Analyses of observations
Altogether, 480 recordings were scheduled for assessment, that is, one recording from 12 intervention arms in 20 patients and 20 control subjects. With respect to assessment of observations, MI-E cycles were edited into three phases of interest: (i) insufflation, (ii) pressure drop (from positive to negative) and (iii) active exsufflation or the voluntary cough with no negative pressure applied. The onset and offset of each phase were observed and defined from the parallel video recording of the MI-E manometer.23 Video recordings were assessed systematically, as described previously,23 by two trained raters (TA and AKB). Main features were described at glottic, supraglottic and hypopharyngeal levels (see online supplementary figure S3). Laryngeal anatomy and motion at rest were evaluated by a senior laryngologist (J-HH).
Statistical analyses
The χ2 test, or Fisher's exact test if expected cell counts were less than five, were applied to assess differences between groups with regard to categorical data. Background data were given as group means with SDs. The number of subjects with the described patterns of laryngeal movements during MI-E was given as group counts and percentages. Statistical analyses were conducted using SPSS V.21.0 (IBM, Armonk, USA). The two-sided significance level was set at 0.05.
Results
Patient characteristics
Of 20 participating patients with ALS, six had limb onset with no bulbar symptoms and 14 had bulbar symptoms (table 1); of these, seven had pseudobulbar (spastic) ALS and seven had progressive bulbar (hypotonic) ALS. Lung-function characteristics in ALS were lower than predicted (table 1). In patients with progressive bulbar ALS, 4/7 subjects had an abnormal epiglottis: three had a juvenile and high-standing epiglottis, and in one patient the epiglottis was considered ‘floppy’. Retention of secretions/sputum was observed in 4/7 patients with progressive bulbar ALS, in 2/7 cases with pseudobulbar ALS, in 2/6 subjects with non-bulbar ALS and in 1/20 healthy controls.
Background characteristics of the study participants (n=40)
Laryngeal response to MI-E
In total, 453 (94%) of 480 scheduled recordings were analysed. Four patients with bulbar symptoms completed only parts of the MI-E protocol due to discomfort from the applied pressures, that is, one patient (progressive bulbar ALS) interrupted the protocol after pressures of ±20 cm H2O (missing 9/12 intervention arms), one patient (pseudobulbar ALS) after ±30 cm H2O (missing 6/12 intervention arms) and two patients (one pseudobulbar and one progressive bulbar ALS) after ±40 cm H2O (both patients missing 3/12 intervention arms). Technical failures led to loss of video recordings in one healthy control at examining pressures of ±40 and ±50 cm H2O (missing 6/12 intervention arms).
In general, the larynx moved downwards during applied insufflation and upwards (cranially) during exsufflation. (See table 2 for overall descriptions and online supplementary video 1 for the laryngeal response in a patient with non-bulbar ALS; online supplementary video 2 in a healthy control; online supplementary video 3 in a patient with progressive bulbar ALS; online supplementary video 4 in a patient with pseudobulbar ALS.) Adequate laryngeal control was defined as described for normal cough in the literature,8 and presented as initial abduction of the true vocal folds (TVF) and aryepiglottic folds (AEF), and thereafter glottic closure with subsequent rapid opening when coughing, abduction of the TVF and AEF followed by sequential closures and/or narrowing in the exhalation phase of the cough.
Description of laryngeal response patterns during the MI-E protocol (n=40)
Supplementary video 1
Supplementary video 2
Supplementary video 3
Supplementary video 4
Response at the glottic level
Observations at the glottic level were not possible in some patients with ALS and bulbar symptoms, because adduction of AEF and/or the hypopharyngeal area obscured the view of TVF, particularly in the high-pressure ranges of 40–50 cm H2O. Observations at the glottic level were based on successful visualisation of MI-E cycles (TVF responses A, B, G, I, M, S, N1, N2 and N3 in figure 1 and online supplementary tables S4, S5 and S6).
Laryngeal response at the glottic level. Figures are percentages of the sample with the described response. *Significant difference between healthy volunteers and patients with ALS. ALS, amyotrophic lateral sclerosis; TVF, true vocal folds.
There were significant differences between patients with ALS and healthy controls with respect to TVF adduction subsequent to the initial abduction during insufflation (response B in figure 1 and in online supplementary table S4) and exsufflation. Varying the instructions (to cough or exhale during negative pressures or to cough without applied negative pressure) did not influence the groups differently (response N1, N2 and N3 in figure 1 and online supplementary table S6).
Response at the supraglottic level
AEF responses are presented as C, D, H, J, O and P (figure 2 and online supplementary tables S4, S5 and S6). Medial rotation of the cuneiform tubercles accompanied by considerable adduction of the AEF was observed during insufflation (initially or subsequent to abduction) in all patients with bulbar ALS (online supplementary table S4 and response C and D in figure 2).
Laryngeal response at the supraglottic level. Figures are percentages of the sample with the described response.*Significant difference between healthy volunteers and patients with ALS. AEF, aryepiglottic folds; ALS, amyotrophic lateral sclerosis.
A retroflex movement of epiglottis (a passive dorsal rotation) was observed to partly occlude the laryngeal inlet in some cases, either as a rapid movement or lasting throughout the insufflation (responses E, K and Q (figure 2 and online supplementary tables S4, S5 and S6)).
Oesophageal opening was observed during insufflation in two patients with progressive bulbar ALS. Both subjects were observed to burp afterwards, suggesting that (part of) the insufflation volume ended up in the oesophagus and stomach instead of the lungs.
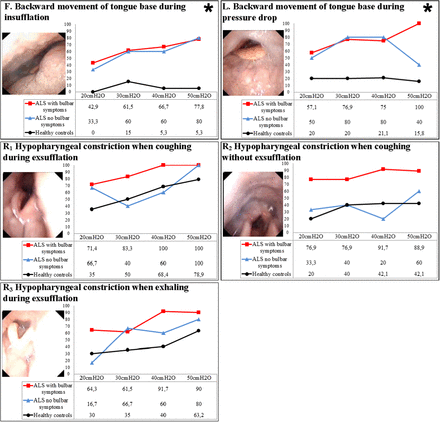
Response at the tongue base and at the hypopharyngeal level
There were significant differences between healthy controls and patients with ALS with regard to backward movement of the tongue base during insufflation and during the pressure drop (responses F and L in figure 3 and online supplementary tables S4 and S5).
Laryngeal response at the tongue base and hypopharyngeal level. Figures are percentages of the sample with the described response. *Significant difference between healthy volunteers and patients with amyotrophic lateral sclerosis (ALS).
Constriction of the hypopharynx during exsufflation was observed in healthy controls and in patients with ALS, regardless of the presence of bulbar symptoms. In patients with ALS and bulbar symptoms, hypopharyngeal constriction was more prominent in those with progressive bulbar paresis. The hypopharynx was totally constricted in 4/7 patients with progressive bulbar paresis and in 1/7 patients with pseudobulbar paresis (responses R1, R2 and R3 in figure 3 and online supplementary table S6).
Differences in laryngeal movements between patients with pseudobulbar and progressive bulbar ALS were not significant. A few significant values were observed between observations of healthy controls and patients with ALS and bulbar symptoms, and between patients with ALS with and without bulbar symptoms. Due to a multiple-testing problem, these results should be interpreted with caution. However, we saw a pattern in comparison between controls and patients with ALS and bulbar symptoms with regard to backward movement of the tongue base during the pressure drop and in subsequent adduction of TVF during exsufflation (see online supplementary tables S4–S6).
Discussion
This is the first study to show that video-recorded flexible laryngoscopy is a feasible method to characterise laryngeal responses throughout MI-E in patients with ALS. Results clearly indicated that MI-E in patients with bulbar symptoms was associated with adduction of supraglottic laryngeal structures during insufflation, and that this seemed to compromise airflow. Backward movement of the tongue base during insufflation, potentially obstructing airflow at the hypopharynx, was more prominent in patients with ALS than in healthy controls. Moreover, patients with ALS, irrespective of subtype, were more likely to adduct the vocal folds during insufflation and exsufflation. Patients with ALS, without bulbar symptoms, could cough in a coordinated way, similar to that seen in healthy controls.
The main strength of this study was provision of important knowledge on a challenging clinical problem achieved using objective and verifiable methods in a population-based sample of patients whose data were compared with those of healthy matched volunteers. The small study cohort was a limitation, complicating statistical handling and rendering the study at risk of particularly type-II errors (ie, failure to detect significant differences that may have been present). A priori power calculation could not be undertaken, because the data distribution was not known when planning the study.24
Transnasal fibre-optic laryngoscopy during ongoing MI-E in patients with ALS has not been described previously, but has been used to describe the larynx during simple tasks (eg, vocalising, spontaneous cough and forced exhalation).25 ,26 We encountered some technical challenges. First, as the larynx moved downwards and upwards during insufflation and exsufflation, dynamic adjustments of the laryngoscope position were required. Sometimes, airway secretions led to poor-quality video recordings, and pretreatment aiming to clear secretions could have been considered. Adduction of supraglottic structures precluded visual access to the vocal folds in some patients.
The present study suggests that adduction of primarily supraglottic laryngeal structures during insufflation may be a critical issue when carrying out MI-E in patients with ALS and bulbar symptoms. Conceivably, the observed adduction prevents lung insufflation before exsufflation, thereby compromising the effect of MI-E. We cannot explain these response patterns, but can only speculate. There is only one abductor muscle in the larynx, the posterior cricoarytenoid muscle, but several small intrinsic adductors.27 Intrinsic laryngeal muscles interact in a complex way during cough, speech and swallowing, but always act in concert. Stimulation of extremely sensitive receptors in the supraglottic larynx usually induces complex adductor reflexes that, for example, prevent foreign bodies from entering the airways.27 This reflex circuit may be hyper-responsive or dysregulated in patients with ALS and, therefore, lead to inappropriate laryngeal closure, comparable with the observations made in patients with Parkinson's disease or brainstem compression.28 ,29 Tomik et al25 observed early dysfunction of the vagal nerve before any clinical signs of bulbar dysfunction in patients with spinal ALS. The observed vocal fold adduction in our study supports this finding.
Differences in the two subtypes of bulbar ALS may influence laryngeal response patterns to MI-E, that is, progressive (hypotonic) versus pseudobulbar (spastic) ALS. In pseudobulbar ALS, laryngeal adduction occurred mainly at the glottic level at relatively high insufflation pressures. It seems reasonable to suggest that positive pressures more easily trigger laryngeal adductor reflexes in a disease that is predominantly spastic. AEF are relatively soft structures provided with only scattered muscle fibres. Therefore, adduction at the supraglottic level could, theoretically, be explained by the Bernoulli principle: increasing airflow initiates negative intraluminal pressures that eventually cause medial collapse.30 This mechanism may conceivably be particularly important in progressive bulbar ALS characterised predominantly by hypotonic paresis. An abnormal high-standing epiglottis may have a practical implication by compromising the laryngeal inlet during insufflation due to retroflex movements caused by the positive pressures, as demonstrated also during treatment with CPAP in patients with obstructive sleep apnoea.31
Hypopharyngeal constriction during exsufflation was observed to varying extents in all study subjects, as well as healthy controls. This finding confirms reports of upper-airway narrowing at pharyngeal and oropharyngeal levels upon application of negative pressures during exhalation in healthy subjects.32–34 This phenomenon has been used to explain the ineffectiveness of MI-E in patients with ALS.35 Sancho et al undertook CT during MI-E at baseline and during exsufflation in three patients with ALS. They reported varying reductions of the lateral diameter at the level of nasopharynx, uvula and pharynx during the exsufflation phase at −40 cm H2O.12 The response during insufflation was not examined. They suggested that MI-E should be carried out by applying a single insufflation followed by a manually assisted cough instead of active exsufflation with negative pressures.35 ,36 Hypopharyngeal constriction during exsufflation was observed in healthy controls and patients with ALS in the present study; so, this phenomenon alone cannot explain treatment failure in bulbar ALS. Moreover, inability to fill the lungs during insufflation because of the observed supraglottic adduction would create a vacuum during the subsequent active exsufflation, and thereby aggravate hypopharyngeal constriction. If this hypothesis is correct, a single insufflation followed by a manually assisted cough cannot help patients with bulbar ALS to cough more effectively, but will be both uncomfortable and unproductive.
The present study suggests that an individual approach to MI-E used in respiratory airway therapy is highly important. Lower positive pressures and airflow combined with longer inspiratory times may contribute to better laryngeal stability during insufflation, perhaps by preventing or reducing the impact of protective laryngeal reflex circuits and the intraluminal suction forces induced by the Bernoulli effect (figure 4). Patients with bulbar insufficiency may, therefore, be more likely to obtain sufficient inspiratory volumes, a situation that would improve the conditions for exsufflation of the lungs. The phasic relationship that exists between the posterior cricoarytenoid muscle and diaphragm is a feature that could, theoretically, be exploited clinically. That is, when the diaphragm contracts, the activity of the posterior cricoarytenoid muscle increases in a coordinated manner due to vagal stimulation, thereby abducting the larynx.27 If the patient is instructed to inhale actively before active insufflation with MI-E, this act would, theoretically, lead to better laryngeal abduction and facilitate airflow. Recently, MI-E devices with a ‘trigger’ function linked to insufflation have become available, and these mechanisms should be studied closely.
A practical algorithm suggesting how to adjust the settings of mechanical insufflation–exsufflation (MI-E) when used to treat patients with amyotrophic lateral sclerosis (ALS) for airway secretion clearance problems, based on observations in the present study.
A better understanding of laryngeal dysfunction as ALS progresses in its various phenotypes can help establish better (and hopefully individually tailored) clinical respiratory treatment strategies for these patients, and perhaps also for other patients with bulbar-innervated muscle dysfunction.
Conclusion
Video-recorded flexible laryngoscopy is a feasible method to characterise laryngeal responses throughout an MI-E protocol in patients with ALS. Treatment failure with MI-E in patients with bulbar symptoms is likely to be caused primarily by laryngeal adduction during insufflation, predominantly at the supraglottic level. This response precludes air-filling of the lungs during insufflation, causing discomfort and subsequent inefficient exsufflation. We propose that individually customised settings for pressure and flow can improve and extend the use of non-invasive MI-E in ALS, and that flexible laryngoscopy can be an efficient tool in this respect in selected patients who do not respond as expected.
Acknowledgments
We extend many thanks to medical photographer Thor-Andre Ellingsen for his valuable help with the video recordings and editing of film clips. We are also very grateful to nurses Gunvor Mo Norstein and Marit Arnevik Renså for coordinating the ALS clinic and contributing to the running of patient examinations.
References
Footnotes
Contributors All authors made a significant contribution to the conception and the design of the article and of the collection, analysis and interpretation of the data, drafting of the article and revising it critically for content and final approval of the version to be published. All authors participate in the research group and are collectively responsible for the final version of this paper.
Funding The Norwegian Centre of Excellence for Home Mechanical Ventilation, Thoracic Department, Haukeland University Hospital, Bergen, Norway and Western Norway Regional Health Authority funded this study.
Competing interests TA has received honoraria of €500 for a lecture in an international conference sponsored by Respironics. Sponsors were not involved and had no impact on the study design, in the collection, analysis and interpretation of data, in writing of the report, nor in the decision to submit the article for publication.
Patient consent Obtained.
Ethics approval Regional Committee for Medical Research Ethics, Western Norway Regional Health Authority, Bergen, Norway.
Provenance and peer review Not commissioned; externally peer reviewed.