Article Text
Abstract
We present the cases of two pregnant women who developed severe respiratory compromise in mid pregnancy, one due to rapidly progressive interstitial lung disease associated with mixed connective tissue disease and one secondary to diffuse alveolar haemorrhage due to antiglomerular basement membrane disease. Both were treated with high-dose steroids followed by pulsed intravenous cyclophosphamide. Both women went onto have live births although one baby was growth restricted and preterm. Neither baby had any evidence of congenital abnormalities.
- Interstitial Fibrosis
- Pulmonary vasculitis
- Systemic disease and lungs
Statistics from Altmetric.com
Case 1
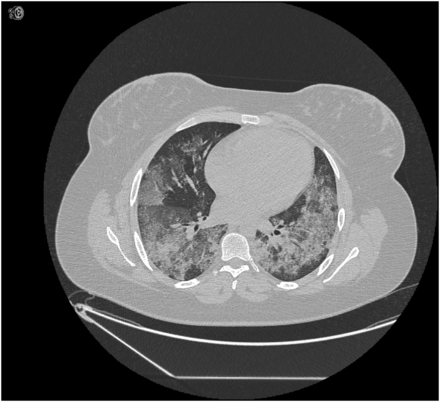
A 22-year-old Caucasian smoker, para 1 with a previous normal vaginal delivery, presented to her local hospital with breathlessness and haemoptysis at 27 weeks gestation. Her pregnancy had been progressing normally. Her haemoglobin was 56 g/L. Her chest X-ray (CXR) showed bilateral diffuse shadowing. She received antibiotics for presumed respiratory infection, blood transfusion and intramuscular corticosteroids to induce fetal lung maturity. She improved after a week and was discharged. She represented 6 days later with similar symptoms, a haemoglobin of 74 g/L. Chest computed tomography (CT) suggested diffuse alveolar haemorrhage (DAH) (figure 1) and computed tomography pulmonary angiography (CTPA) excluded pulmonary embolism (PE). Treatment with oral prednisolone 30 mg/day resulted in no improvement and she was requiring 40% O2 to maintain saturations above 94%. She was transferred to our unit for further investigation and management. On arrival, pO2 was 9.6 kPa on 60% O2, she was tachycardic (130 bpm) and tachypnoeic with a respiratory rate of 30. Urinalysis showed 1+ protein and 1+ blood. Haemoglobin was 85 g/L, white cell count was 15.4×109/L (neutrophils 12.5×109/L), platelets were 348×109/L, clotting screen was normal, erythrocyte sedimentation rate (ESR) was 40 mm/h and C reactive protein (CRP) was 59 g/L. Electrolytes and creatine kinase were normal and serum creatinine was 53 μmol/L. Antinuclear antibodies, double-stranded DNA (dsDNA), extractable nuclear antigens, rheumatoid factor, anti-Cyclic Citrullinated Peptide (CCP), antineutrophil cytoplasmic antibody (ANCA) and viral screen were negative. Complement levels were normal but antiglomerular basement membrane (GBM) antibodies were positive (17, normal range 0–7). A diagnosis of DAH secondary to anti-GBM antibody disease (Goodpasture's syndrome) was made.
CT axial slice of patient 1 showing diffuse alveolar haemorrhage.
She was treated with 1 g of intravenous methyl prednisolone for three consecutive days and daily plasma exchange of 4 L (1.0 body volume) with fresh frozen plasma as replacement for 7 days. She improved rapidly after 1–2 days, with reduction in heart rate, respiratory rate and oxygen requirements. Anti-GBM fell to 4.8. She then received intravenous cyclophosphamide (1.2 g). Her haemoptysis stopped on the third day after transfer and her CXR showed complete resolution on the fifth day. She was commenced on prophylactic low molecular weight heparin, which was continued until discharge from hospital. She was discharged 10 days following transfer and taking a maintenance dose of 80 mg (1 mg/kg) of prednisolone. She developed gestational diabetes mellitus (GDM) necessitating insulin therapy with humulin I once daily and premeal novorapid. She received two further doses of cyclophosphamide at two weekly intervals at which point the anti-GBM antibody titre was undetectable, the ESR was 13 mm/h and the CRP was 2 g/L. Prednisolone dose was gradually weaned to 25 mg daily. She had a normal vaginal delivery following induction of labour at 37 weeks because of GDM. She remains asymptomatic and with normal renal function and no recurrence of pulmonary haemorrhage.
Case 2
A 34-year-old Asian never smoker, para 1 with a previous normal vaginal delivery, presented to her local hospital with an 18-month history of progressive breathlessness at 20 weeks gestation. Following a rapid deterioration, she was ventilated and transferred to the intensive care unit. Her CXR showed widespread infiltrates. She received broad-spectrum antibiotics and 1 g of intravenous methylprednisolone on three consecutive days followed by 30 mg of oral prednisolone with a good response and was extubated. She was transferred to our unit for further investigation and management. On arrival, her pO2 was 9.6 kPa on 60% O2, she was tachycardic 120 bpm and tachypnoeic with a respiratory rate of 24. Haemoglobin was 115 g/L, white cell count was 12.7×109/L (neutrophils 10.9×109/L, lymph 0.9×109/L), platelets were 457×109/L, clotting screen was normal, ESR was 53 mm/h and CRP was 9 g/L. Electrolytes and creatine kinase were normal and serum creatinine was 56 μmol/L. Microbiology was negative.
On further questioning, she also had a history of inflammatory joint pain, myalgia and muscle fatiguability, Raynaud's phenomenon and constitutional fatigue. Investigations showed a strongly positive ANA (1/1280) with U1RNP positivity. dsDNA, rheumatoid factor and ANCA were negative and complement levels were normal. A CT scan showed bibasal fibrotic change with honeycombing, areas of peripheral consolidation and groundglass opacities bilaterally (figure 2). A diagnosis of an acute exacerbation of an underlying fibrotic nonspecific interstitial pneumonia (NSIP) associated with mixed connective tissue disease was made. Given the underlying disease progression and ongoing respiratory compromise, she was treated with 6 pulses of 500 mg of intravenous cyclophosphamide at 2 weekly intervals and prednisolone 40 mg daily with a slow taper. Her breathlessness improved and her oxygen saturations improved to 96% on air. She was discharged 8 days following transfer. Her CXR showed significant improvement with residual bibasal reticulation. She was started on azathioprine as maintenance therapy after cyclophosphamide induction was complete and remained well throughout the rest of her pregnancy. She had a caesarean section at 33+4 weeks gestation for reduced fetal movements, fetal growth restriction and reversed diastolic flow on umbilical Dopplers. The baby boy weighed 1.11 kg (less than 1st centile) and was admitted to the neonatal unit. The neonatal full blood count performed on the day of delivery showed a haemoglobin of 114 g/dL, white cell count was 6.4×109/L (neutrophils 1.9×109/L, lymphocytes 3.5×109/L) and platelets 175×109/L. After initial support, he did well requiring no further respiratory support and remained on the unit to establish enteral feeds. He was transferred to his local hospital aged 8 days. He continued to do well and was discharged from the local unit aged 4 weeks. The mother remains asymptomatic with no progression of her interstitial lung disease (ILD).
CT axial slice of patient 2 showing fibrotic NSIP.
Conclusion
A systematic review1 reported only eight cases of de novo anti-GBM antibody disease in pregnancy. Neither bronchoalveolar lavage nor kidney biopsy was performed in our case as the combination of DAH with positive anti-GBM antibodies was deemed sufficient to make the diagnosis without the risk of more invasive procedures.
The options for induction/consolidation therapy in both these cases were cyclophosphamide or rituximab. Given the gestational age of the pregnancy (28 and 20 weeks), cyclophosphamide was thought to be preferable, since at these gestations there is no risk of teratogenesis, and data from chemotherapy given in pregnancy for malignancy2 suggest no adverse effects on the fetus or neonate.3 Rituximab may lead to suppression of fetal neonatal B cells for up to 6 months. Thus, it may be used in early pregnancy but ideally not within 6 months of delivery. Both patients were counselled regarding the risks of premature ovarian failure and bone marrow suppression with cyclophosphamide use.
The lesson from both these cases is that appropriate management of inflammatory lung disease causing respiratory failure in pregnancy should not be compromised because of the pregnancy nor the pregnancy terminated early in order to treat the condition. Good long-term outcomes for both mother and baby are possible with multidisciplinary team work and discussion regarding appropriate treatments in pregnancy.
Acknowledgments
We thank the multidisciplinary teams that helped care for both patients.
Footnotes
Contributors All authors contributed to the conception of the case reports and management of the patients. CN-P and SA wrote the first draft. All authors revised subsequent versions and approved the final version.
Competing interests None declared.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.