Article Text
Abstract
Background Hospitalisation for acute exacerbations of COPD is associated with high risk of readmission. However, no tool has been validated to stratify patients at discharge for risk of readmission.
Aim To evaluate the ability of the 4 m gait speed (4MGS), a surrogate marker of frailty, to predict risk of future readmission in hospitalised patients with an acute exacerbation of COPD (AECOPD).
Methods 213 patients hospitalised with an AECOPD were recruited prospectively. 4MGS was measured on day of discharge. Logistic regression models were used to assess the association between 4MGS and readmission at 90 days after discharge.
Results Baseline characteristics of the cohort: 52% men; mean age 72 years; median FEV1 35%predicted. Mean (SD) 4MGS at hospital discharge was 0.61 (0.26) ms−1. Significant increased rates of all-cause readmission at 90 days were seen across quartiles of decreasing 4MGS (Q4 fastest: 11.5%; Q3: 20.4%; Q2: 30.2%; Q1 slowest: 48.2%; ptrend<0.001). Compared with Q4, those in the slowest 4MGS quartile had unadjusted ORs (95% CIs) for 90-day readmission of 7.12 (2.61 to 19.44) for the whole cohort and 11.56 (3.08 to 43.35) in those aged 65 or over. A multivariate model incorporating 4MGS, Charlson Index, hospital admission in past year, FEV1%predicted and number of exacerbations in past year in those aged 65 or over predicted 90-day readmission with a C-statistic of 0.86.
Conclusions The 4MGS, a surrogate marker of physical frailty, independently predicts the risk of readmission in older patients hospitalised for acute exacerbation of COPD.
Trial registration number NCT01507415.
- COPD Exacerbations
Statistics from Altmetric.com
Video abstract
Key messages
What is the key question?
Does the 4 m gait speed (4MGS), a simple surrogate marker of physical performance, mobility and frailty, predict readmission following an acute exacerbation of COPD?
What is the bottom line?
The 4MGS at hospital discharge independently predicts the risk of readmission in those hospitalised for acute exacerbation of COPD in older patients.
Why read on?
This is the first study in COPD to provide evidence that the 4MGS is a feasible test in patients hospitalised for acute exacerbation of COPD, and may be of value in risk stratifying patients at risk of readmission. This may help clinicians individualise post-discharge care.
Introduction
Hospitalisations for acute exacerbations of COPD (AECOPD) represent a major event in the natural history of the disease, and account for up to 70% of total COPD costs.1 ,2 Hospital admissions are associated with increased mortality, and high readmission rates with 22.6% readmitted within 30 days in the USA3 and 31.4% readmitted to hospital within 90 days of discharge in the UK.4 Efforts to reduce hospital readmissions are therefore high on both political and patient agendas.5 Stratifying patients for risk of readmission may help clinicians individualise post-discharge care, and thus potentially reduce healthcare costs.
Although previous studies have examined potential risk factors for readmissions, some were retrospective analyses of routinely collected datasets, or focused on indices of admission severity or socio-demographic variables that are not amenable to intervention.6 Physical performance and mobility are of particular interest as they are potentially amenable to intervention through exercise modalities such as early peri-exacerbation pulmonary rehabilitation.7 ,8 The 4 m gait speed (4MGS) is a simple measure of physical performance, and a surrogate marker of physical frailty that has been shown to predict adverse outcomes such as future hospitalisations, disability, nursing home admission, falls and mortality in autonomous community-dwelling older people.9 Due to its simplicity and need for little space, the 4MGS is feasible in most clinical settings,10 and acceptable to even very frail patients.11 In patients with COPD, the 4MGS has been recently shown to have excellent test–retest and inter-observer reliability, to correlate significantly with exercise capacity, health status and dyspnoea,12 and to be responsive to both intervention and longitudinal change.13 Recently, gait speed has been demonstrated to be feasible in the acute setting.14 In unselected acutely ill older patients, Ostir et al14 showed that gait speed was independently associated with length of hospital stay and odds of discharge to home.
The aim of our study was to evaluate the ability of the 4MGS measured at hospital discharge to predict the risk of readmission in patients hospitalised with an AECOPD. We hypothesised that patients with slower gait speed at hospital discharge would have an increased risk of all-cause non-elective readmission at 90 days.
Methods
Participants were recruited prospectively from acute medical wards at Hillingdon Hospital, UK. All participants gave written consent and the study was approved by the National Research Ethics Committee (11/LO/1250). The study was registered with the UK Clinical Research Network Portfolio (ID:11212) and ClinicalTrials.gov (NCT01507415).
Inclusion criteria were a diagnosis of COPD according to GOLD guidelines;15 hospital admission with a primary diagnosis of AECOPD as determined by the duty clinician and confirmed by the local specialist respiratory team; resident in the borough of Hillingdon; and age over 35 years. Need for hospital admission was determined by the duty emergency department clinician. Admission was defined as a medical ward stay outside of the emergency department of greater than 4 h, and expected to be greater than 24 h duration. Patients with known COPD but with chest radiograph changes of consolidation were also included if the admission necessitated a change in or addition to their usual COPD treatment.
Exclusion criteria were patients with unstable cardiac disease; inability to walk 5 m unassisted on day of hospital discharge; predominant neurological limitation to walking (eg, significant hemiplegia); severe cognitive dysfunction (score of 5 or below on 10-item abbreviated mental test score);16 or poor English such that informed consent could not be obtained.
All patients received a minimum 7 days of oral corticosteroids (prednisolone 30 mg daily), and were treated initially with nebulised bronchodilators. Antibiotics were given for presumed infective exacerbation at the judgement of the supervising clinical team. All patients were supported by a nurse-led COPD outreach team for 2 weeks, who provided telephone support and home visits as necessary.
Measurements
All measurements were carried out within 24 h before hospital discharge. 4MGS was performed as previously described.12 Participants were allowed to use their normal walking aids (eg, stick or frame) and oxygen if required. Socio-demographic details, smoking pack year history, number of self-reported exacerbations (necessitating change in medication) and hospitalisations (derived from medical records) in the last year and, details regarding the index admission were recorded. FEV1 was measured17; if flow volume loops were non–reproducible or the patient declined spirometry during hospital admission, their last documented FEV1 was recorded. Other measurements included respiratory disability (Medical Research Council (MRC) Dyspnoea score),18 help with activities of daily care (Katz Index),19 self-reported daily physical activity (modified Minnesota Leisure-time Physical Activity Questionnaire),20 health status (St George's Respiratory Questionnaire, SGRQ),21 comorbidities (Charlson Index)22 and a surrogate marker of admission severity (DECAF score incorporating the five strongest predictors of in-hospital mortality (extended MRC Dyspnoea Score, Eosinopenia, Consolidation, Acidaemia, atrial Fibrillation)).18 Indices of Multiple Deprivation 2010 were derived from home postcodes.23
The primary outcome was all-cause non-elective hospital readmission in the 90 days after hospital discharge. Data were obtained from patients and their families and corroborated by hospital and general practice records by a researcher blinded to the measurements made at discharge. The cause of readmission was identified for each episode on the basis of the discharge summary.
Data analysis
Descriptive statistics were used to summarise participant characteristics. We investigated associations between categorical variables using χ2 tests or, when numbers were low, Fisher's exact test. Between-group differences were assessed using Student t tests or Mann–Whitney U tests (non-normally distributed data) for continuous variables. For comparing more than two groups, ANOVA or Kruskal Wallis was used.
Univariable logistic regression was used to assess the association between 4MGS (and other plausible physiological measures) and all-cause 90-day readmission. 4MGS was considered as quartiles and also as a continuous variable (per 0.1 m/s decline). All variables associated with readmission where p≤0.15 were considered in the multivariable model. A backwards stepwise procedure was used and variables remained in the model if p<0.20. The interaction between age and 4MGS (both as continuous variables) was considered in the final model; modification was observed (p=0.013) and therefore a subgroup analysis was performed in those aged 65 years or older. This cut-off was chosen as previous large epidemiological studies have validated the prognostic value of the 4MGS in this age category only.24 To evaluate the ability of 4MGS to predict time to readmission, we used a Kaplan–Meier analysis with significance assessed with the use of the log-rank test for trend. In addition to the univariable and multivariable models, a number of other models were considered and area under the curve (AUC or C-statistic) was used to assess discrimination of models. Hosmer–Lemeshow goodness of fit tests were also conducted on all models. A p value of 0.05 was considered statistically significant. Data analyses and graphical presentations were performed using STATA V.13 (StataCorp LP, Texas, USA).
Sample size
Sample size was based on receiver operating characteristic (ROC) analysis and the area under the ROC curve with 90-day readmission status as a binary outcome predicted by a multivariable model incorporating 4MGS. Assuming a 25% readmission rate (ie, 3 non-admitted controls for every admitted case), 204 patients (51 cases and 153 controls) would be needed to show that the C-statistic is above 0.6 where the true value is 0.73.7 Assuming a potential 10% lost to follow-up, the recruitment target was 225 patients.
Results
A recruitment flowchart is shown in figure 1. Three hundred and seventy-eight patients were screened for eligibility, of whom 226 patients consented to take part in the study. Six patients were discharged before 4MGS was performed, four withdrew their consent, one died in hospital on the day of discharge, and one became acutely confused. A further patient died suddenly and unexpectedly at home 18 h after discharge. Therefore 213 patients were included in the final analysis (figure 1).
CONSORT diagram of patient flow through the study. AECOPD, acute exacerbation of COPD.
Baseline characteristics of patients as a whole and stratified by quartiles of 4MGS are shown in table 1 and online supplementary table E1. Mean (SD) 4MGS was 0.61 m/s (0.26). Patients with lower gait speed at discharge were significantly older, had longer lengths of stay, worse respiratory disability (MRC), higher comorbidity burden (Charlson Index), poorer health status (SGRQ), and higher DECAF scores (table 1). Only 7% of patients underwent early post-hospitalisation pulmonary rehabilitation (see online supplementary table E1), which had no significant effect upon subsequent analyses of readmission.
Baseline characteristics of the total sample and stratified by quartiles of gait speed
Readmission risk
In total, 59 patients (27.7%) were readmitted within 90 days of hospital discharge (47 for respiratory and 12 for non-respiratory causes). Baseline characteristics of those admitted and not admitted within 90 days are summarised in table 2.
Baseline characteristics stratified by readmitted or not readmitted within 90 days
For the primary outcome, the risk of 90-day all-cause readmission decreased as 4MGS increased (Q1 (slowest) 48.2%, Q2 30.2%, Q3 20.4%, Q4 (fastest) 11.5%; ptrend<0.001) (see online supplementary figure E1). In those ineligible for the study due to inability to walk 5 m unaided (n=33), 90-day readmission rate was 54.5% (n=18).
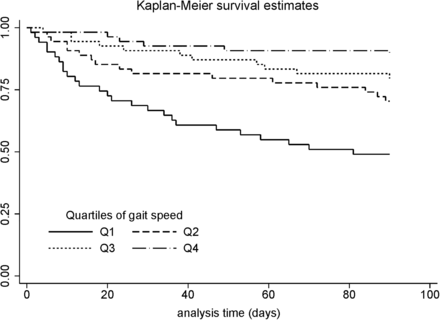
For the secondary outcome, Kaplan–Meier curves (figure 2) demonstrated reduced time to 90-day all-cause non-elective readmission with slower 4MGS quartiles (log-rank test for trend: p<0.001).
Kaplan–Meier curve demonstrating time to 90-day all-cause non-elective readmission according to 4MGS quartile (Q1≤0.40 m/s; Q2=0.40–0.59 m/s; Q3=0.60–0.79 m/s; Q4≥0.80 m/s). 4MGS, 4 m gait speed.
In total, the cohort occupied 601 emergency hospital bed days in the 90 days after hospital discharge with decreasing cumulative hospital bed days with faster gait speeds (Q1 (slowest) 316; Q2 157; Q3 106; Q4 (fastest) 22 days).
Univariable analysis for risk of 90-day readmission in the whole cohort is demonstrated in online supplementary table E2. Compared with those with the fastest 4MGS (Q4), those in the slowest 4MGS quartile (Q1) had an unadjusted OR (95% CI) of 7.12 (2.61 to 19.44). Using gait speed as a continuous measure, there were increasing odds of readmission at 90 days for each 0.1 m/s decline in gait speed (OR 1.30 (1.14 to 1.48); p<0.001)). Results of the multivariable analysis for the whole cohort confirmed that 4MGS was an independent risk factor for 90-day readmission. The interaction term between age and 4MGS was added to the final model and was significant (p=0.013) and therefore subgroup analysis was performed.
In patients aged 65 years or over, 53 (32.3%) patients were readmitted within 90 days. Univariable analysis in this subgroup demonstrated decreasing risk of 90-day readmission with faster gait speed (OR (95% CI): Q1 11.56 (3.08 to 43.35); Q2 4.38 (1.15 to 16.70); Q3 2.8 (0.69 to 11.41); Q4 1 (reference); p<0.001) (table 3). In the multivariable model (table 4) 4MGS remained an independent risk factor for 90-day readmission (OR 1.43 (1.13 to 1.80) per 0.1 m/s decline in 4MGS). The association between 4MGS (0.1 m/s decline) and all cause readmission was not significant in those aged below 65 (OR (95% CI) 0.90 (0.67 to 1.22), p=0.490), although numbers of readmissions were low (n=6 (12.2%)) and therefore no further analysis was performed.
Univariable logistic regression predicting all-cause readmission at 90 days in patients 65 years or older (n=164)
Multivariable logistic regression predicting all-cause readmission at 90 days in patients 65 years or older (n=164)
Predicting readmission
ROC plots and C-statistic of various models to predict hospital readmission at 90 days, in those 65 or over, are shown in figure 3. A model incorporating 4MGS and hospital admissions in the last year had a C-statistic of 0.78 (acceptable discrimination). The full model incorporating all independent risk factors identified in the multivariable analysis had excellent discrimination with a C-statistic of 0.86. The C-statistic of the full model without gait speed was 0.82, implying that 4MGS adds value as a predictive marker.
Receiver operating characteristic curves demonstrating the ability of univariable and multivariable models to predict 90-day readmission in patients 65 years or older. Model 1=age; Model 2=one or more admissions in last year; Model 3=4 m gait speed; Model 4=4 m gait speed+one or more admissions in last year; Model 5=multivariable model (see table 4; gait speed, Charlson Index, one or more admissions in the previous year, number of exacerbations in the previous year, length of stay, physical activity and health status).
Discussion
This is the first study to prospectively test whether objectively measured physical performance at discharge predicts all-cause readmission in patients hospitalised with AECOPD. We have demonstrated that slower usual 4MGS on the day of discharge is associated with significantly increased odds of all-cause non-elective hospital readmission at 90 days, increased post-discharge hospital bed days and reduced time to readmission. Consistent with our findings, those ineligible for the study due to inability to complete the 4MGS had even higher readmission rates. Given the simplicity of the 4MGS, this is a potentially useful tool to risk stratify older patients with COPD in the acute setting, and individualise discharge planning and post-discharge care.
Due to the high readmission rates associated with hospitalisations for AECOPD,3 ,4 there is considerable interest in stratifying patients at increased risk of readmission, and then intervening effectively to reduce that risk. Several lines of indirect evidence suggest that surrogate markers of poor physical functioning and frailty may increase the risk of readmission following hospitalisation for AECOPD. Previous studies have demonstrated that functional limitation (need for help with self-care prior to admission) is an independent risk factor for increased risk of hospital readmission in patients with COPD.25 ,26 Similarly, Garcia-Aymerich and colleagues20 demonstrated that self-reported higher level of usual physical activity in patients admitted for AECOPD were associated with reduced risk of readmission. In a small cohort of patients with COPD discharged from hospital (n=21), Emtner et al27 demonstrated that exercise capacity as measured by the incremental shuttle walk (ISW) at 4–6 weeks after discharge was a reliable predictor of subsequent hospital readmission over 12 months.
To our knowledge, this is the first study to prospectively determine the prognostic value of physical performance objectively measured at hospital discharge in patients with COPD. Although field walking measures, such as the ISW and 6-min walk (6MW), have been well validated in patients with COPD,28 these tests are performed at or close to peak oxygen consumption. They have to be repeated after adequate rest due to learning effect, and the 6MW requires a quiet, uninterrupted walking course of 30 m.28 Although these tests are feasible in stable patients with COPD, even in those with respiratory failure, they may be less acceptable to unwell hospitalised patients and may not be logistically possible in some acute hospital settings. Consequently, we used the 4MGS, which is well established in gerontology as a simple surrogate marker of physical performance, mobility, sarcopenia and frailty.29–31 In our study, more than 90% of potentially eligible patients with COPD were able to perform a 4MGS on the day of discharge. The results from our current study also corroborate recent findings in older patients admitted with a wide range of medical diagnoses to an acute care elderly unit. Ostir et al14 demonstrated that a faster usual gait speed (measured over 8 ft on the day of admission) was strongly associated with shorter length of stay, whilst the inability to complete the walk, or a gait speed less than 0.40 m/s, had significantly decreased odds of discharge to home. In a large multicentre cohort of acutely ill, hospitalised male veterans, each 0.10 m/s increase in baseline gait speed (measured over 50 ft) was associated with fewer hospitalisation days and healthcare costs in the following year.32
In the management of people with long-term conditions such as COPD, there has been much interest in identifying those who would benefit from more targeted care approaches from those who do not, thus enabling health planners to channel both financial and clinical resources appropriately. The FEV1 was similar across all quartiles of gait speed, suggesting that readmission was more closely related to multi-morbidity and frailty than to severity of the underlying disease. Therefore gait speed may provide a more holistic viewpoint of a patient's current status.
Existing risk stratification models for long-term conditions have a major disadvantage in that the results are not available to practitioners to use for real-time clinical decisions. The simplicity of the gait speed means it could be incorporated easily into routine clinical practice, allowing clinicians to identify a phenotype associated with increased healthcare usage. We observed a more than 14-fold increase in hospital bed days in the slowest 4MGS quartile group compared with the fastest quartile (316 vs 22 days) in the 90 days following discharge. Recognising frail patients at increased risk of adverse outcomes may aid the initiation of palliative care discussions as part of individualised post-discharge care. Tailoring post-discharge health and social support may pave the way for a paradigm shift from existing reactive care models to a more preventative/proactive care strategy.
Cummings et al31 recently postulated that routine identification of ‘dismobility’ defined as a slow 4MGS may help improve the clinical care, research and regulatory approval of pharmacological interventions to improve mobility, including anabolic agents.33 As well as stratifying patients for trial entry, 4MGS is a continuous measure that is a potentially useful outcome marker.34 We recently demonstrated that 4MGS is responsive to the effects of outpatient pulmonary rehabilitation.13 Unlike baseline lung function severity, comorbidity burden, or previous hospital admissions, physical performance is potentially amenable to treatment.13 By demonstrating the association between poor physical performance, frailty and increased readmission risk, this may encourage clinicians and healthcare planners to recommend interventions that improve or maintain physical performance, such as physical activity interventions,35 supervised exercise training in the form of pulmonary rehabilitation,7 or comprehensive geriatric assessment.36 It was noteworthy that only 7% of our cohort underwent peri-hospitalisation pulmonary rehabilitation, in line with recent data from a systematic audit.37
There were limitations associated with our study. First, this was a single-centre study and the findings need to be corroborated by further studies in COPD-specific populations in different healthcare systems. However, we believe that the recruiting hospital was typical of many other UK hospitals, and observed readmission rates were similar to those seen in national audits in the UK and the USA. Second, as 4MGS was the measure of interest, patients unable to walk 5 m independently were excluded, and hence our study focused only on ambulatory patients. However, we did observe high readmission rates in those ineligible for the study due to inability to complete a 4MGS, in line with the study of Ostir et al.14 Third, our cohort had only a small number of younger patients hospitalised with COPD, and larger studies will be needed to demonstrate whether 4MGS also has prognostic value in younger patients. Finally, 4MGS was only measured in the 24 h before hospital discharge—we were unable to comment on whether regular measurement of 4MGS during a hospital admission could influence medical decision to discharge, an area amenable to further research.
In summary, the 4MGS at hospital discharge, a simple surrogate marker of physical performance, mobility and frailty, independently predicts the risk of readmission in those hospitalised for AECOPD, especially in older patients.
Acknowledgments
The authors would like to acknowledge the Hillingdon COPD Outreach Team for their help in identifying potential participants.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online supplement
Footnotes
Contributors WD-CM is guarantor for the study and takes responsibility for the decision to submit this manuscript. All authors had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: WD-CM; acquisition of data: SSCK, SEJ, BMH, MJD; analysis and interpretation of data: SSCK, WD-CM, SJS, WB, PC, MIP; drafting of the manuscript: SSCK, WD-CM, SJS, WB; critical revision of the manuscript for important intellectual content: WD-CM, SSCK, SJS, WB, PC, MIP, BMH, MJD, JLC, CMN.
Funding SSCK is supported by the Medical Research Council (MRC). WD-CM is supported by a National Institute for Health Research Clinician Scientist Award (CS/7/007), a MRC (UK) New Investigator Research Grant (G1002113), a National Institute for Health Research Clinical Trials Fellowship (NIHR-CTF-01-12-04). He is also part funded by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for Northwest London. This project was undertaken at the NIHR Respiratory Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. JLC, SEJ and MIP's salaries are wholly or part funded by the NIHR Biomedical Research Unit. The views expressed in this publication are those of the authors and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health.
Competing interests MIP has received personal reimbursement for lecturing or consultancy regarding muscle function in COPD from Novartis and Philips Respironics. He discloses institutional reimbursement for consultancy from GSK, Novartis, Regneron, Lilly, Biomarin and BI and institutional agreements to conduct research with GSK, Novartis, AZ and Philips Respironics.
Ethics approval National Research Ethics Committee (11/LO/1250).
Provenance and peer review Not commissioned; externally peer reviewed.