Article Text
Abstract
Background Effects of prenatal and postnatal exposure to air pollution on lung function at preschool age remain unexplored. We examined the association of exposure to air pollution during specific trimesters of pregnancy and postnatal life with lung function in preschoolers.
Methods Lung function was assessed with spirometry in preschoolers aged 4.5 years (n=620) participating in the INfancia y Medio Ambiente (INMA) cohort. Temporally adjusted land use regression (LUR) models were applied to estimate individual residential exposures to benzene and nitrogen dioxide (NO2) during specific trimesters of pregnancy and early postnatal life (the first year of life). Recent and current (1 year and 1 week before lung function testing, respectively) exposures to NO2 and nitrogen oxides (NOx) were also assessed.
Results Exposure to higher levels of benzene and NO2 during pregnancy was associated with reduced lung function. FEV1 estimates for an IQR increase in exposures during the second trimester of pregnancy were −18.4 mL, 95% CI −34.8 to −2.1 for benzene and −28.0 mL, 95% CI −52.9 to −3.2 for NO2. Relative risk (RR) of low lung function (<80% of predicted FEV1) for an IQR increase in benzene and NO2 during the second trimester of pregnancy were 1.22, 95% CI 1.02 to 1.46 and 1.30, 95% CI 0.97 to 1.76, respectively. Associations for early postnatal, recent and current exposures were not statistically significant. Stronger associations appeared among allergic children and those of lower social class.
Conclusions Prenatal exposure to residential traffic-related air pollution may result in long-term lung function deficits at preschool age.
- Lung Physiology
- Paediatric Lung Disaese
- Respiratory Measurement
Statistics from Altmetric.com
Key messages
What is the key question?
Does exposure to outdoor air pollution during the prenatal and the early postnatal period impact lung function at preschool age?
What is the bottom line?
Exposure to higher levels of benzene and NO2 during the second trimester of pregnancy was associated with clinically relevant deficits in lung function at preschool age.
Why read on?
This is the first prospective population-based study evaluating the impact of air pollution acting through different windows of susceptibility for lung development including specific trimesters of pregnancy and first years of life on lung function at preschool age.
Introduction
Adverse effects of air pollutants on lung function in school-age children and adolescents have been extensively highlighted in both cross-sectional1–4 and longitudinal studies.5–9 However, susceptibility exposure windows during in utero lung development and impact on preschool lung function remain unexplored. In humans, respiratory airways development occurs during the second and third trimesters of pregnancy and continues until 3 years of age.10 ,11 During these early stages of development and rapid growth, immature lungs may be highly vulnerable to permanent harmful effects of environmental factors including air pollutants.12 ,13
Preschool children represent one of the major challenges in lung function assessment; however, evaluating lung function in this age group is important for clinical reasons and also due to the considerable growth and development of the respiratory system that occurs. To date, few studies have assessed lung function at preschool age—most of them assessing airway resistance—in relation to exposure to air pollution early in life with inconsistent results.7 ,8 Furthermore, very little work has been done on assessing the impact of exposure to air pollution during the prenatal period on lung function later in life. Only a small study conducted among 176 preschoolers of non-smoking mothers showed significant deficits in spirometric lung function parameters at age 5 years in relation to higher 48 h personal measurements of fine particulate matter during pregnancy.14
The limited epidemiological evidence on prenatal and early postnatal exposure to air pollutants on lung function effects warrants further investigation to understand the full impact of air pollution on lung development and growth. Furthermore, new evidence on adverse effects of air pollution exposure on lung function at preschool age, a more objective measurement, will support previous findings on associations of air pollution with subjectively reported respiratory symptoms.15 Here, we aimed to examine the associations between exposure to outdoor air pollution during specific trimesters of pregnancy and postnatal life with lung function at preschool age.
Methods
Study population
The INfancia y Medio Ambiente (INMA) Project is a population-based mother–child cohort study set up in several geographic areas in Spain.16 For the present study, data came from two areas of study: Sabadell and Gipuzkoa. Pregnant women (n=1295, 657 from Sabadell and 638 from Gipuzkoa) were recruited at their first routine antenatal care visit in the public health centre or referral hospital, from 2004 to 2008. Inclusion criteria were: ≥16 years of age, intention to deliver at the reference hospital, no problems of communication, singleton pregnancy, and no assisted conception. A total of 1175 (91%) children had available data on exposure to air pollution, and were eligible (602 from Sabadell and 573 from Gipuzkoa) (see online supplementary figure S1). The study was approved by the ethical committees of the centres involved in the study, and written informed consent was obtained from the parents of all children.
Residential air pollution exposure assessment in pregnancy and postnatal lifetime periods
We developed area-specific land use regression (LUR) models of benzene and nitrogen dioxide (NO2) to estimate residential-based exposures during specific trimesters of pregnancy and early postnatal life (during the first year of life) as previously described.15 ,17 Ambient levels of benzene and NO2 were measured with passive samplers (Radiello, Fundazione Salvatore Maugeri, Padua, Italy) distributed over the study areas according to geographic criteria, taking into account the expected pollution gradients and the distribution of the residences of the women. The samplers remained exposed during several periods of 1 week each as previously described.18 ,19 Further information is given in online supplementary table S1. Measurements taken in the different sampling campaigns were averaged to represent annual mean levels in each study area. Potential predictor variables, such as traffic indicators, surrounding land use, topography, and population density were derived in the geographic information system (GIS) ArcGIS 9.1 (ESRI, Redlands, California, USA). Multiple linear regression models were built using a supervised forward stepwise procedure. Traffic-related variables, altitude and land uses (urban, industrial, or agricultural), were the main predictor variables in the final LUR models. Models explained 75% and 51% of the variability in measured NO2 levels, and 72% and 44% of the variability in measured benzene levels, depending on the study area (see online supplementary table S1). Residential addresses were geocoded (Sabadell area n=608 and Gipuzkoa area n=573) using mapping applications from the regional governments. LUR models were applied to predict outdoor levels of both pollutants at the residential addresses. NO2 estimates were temporally adjusted by using the daily NO2 levels obtained from the monitoring network stations covering the study areas. Due to the lack of benzene measurements in many stations, and high missing data in those stations measuring benzene, we used the pollutant that exhibited the highest correlation with benzene for temporal adjustment (see online supplementary table S1), as in previous studies.15 ,17 ,18 For women and infants who changed their residential address during the study period, we calculated an average exposure estimate weighted by the time spent at each residence. We derived individual exposures to benzene and NO2 during pregnancy by multiplying the LUR estimate by the ratio between the average concentrations measured at the fixed stations over the woman's pregnancy period and over the whole air pollution sampling period. We applied the same procedure to estimate exposures during each trimester of pregnancy and the first year of life.
To assess recent and current individual exposure to outdoor air pollution, we estimated NO2 and nitrogen oxides (NOX) levels at home addresses using LUR modelling developed within the European Study of Cohorts for Air Pollution Effects (ESCAPE) project framework.20 ,21 Briefly, home addresses of participants were geocoded at postal address including residential changes from pregnancy to age 4.5 years. Information on exact dates when participants switched address was also collected. LUR models were based on real air pollution measurements spread out within the study area together with GIS variables on traffic, population density, land use, elevation and topography to predict annual concentration levels at unmeasured locations. Data from routine monitoring stations were used to temporally adjust the long-term exposures for each address to the exactly temporal window desired. We estimated the spatiotemporal exposure at each address and period for which each participant lived in. Recent and current exposure to air pollution were estimated as average of temporally adjusted spatial exposure at children's current address during 1 year and 1 week, respectively, before the lung function testing.
Lung function assessment
At age 4.5 years, 967 children with prenatal air pollution assessment were invited for lung function testing, and 817 (84%) participated. A trained nurse performed the pulmonary function tests. Spirometry test was performed by using a portable spirometer (EasyOne, NDD Medical Technologies, Zürich, Switzerland) with computerised data acquisition software in a portable computer after regular calibration. Lung function was measured according to American Thoracic Society and European Respiratory Society guidelines.22 A total of 197 children had no technically acceptable testing and were excluded. Finally, 620 children had at least 1 acceptable manoeuvre and were eligible. The following lung function parameters were investigated: FVC, mL, FEV1, mL, forced expiratory flow between 25% and 75% of FVC (FEF25–75, mL/s) and peak expiratory flow (PEF, mL/s). The best FVC and best FEV1 were recorded, whereas FEF25–75 and PEF were derived from the best curve, defined as the greatest sum of FVC and FEV1. A reproducible test was defined as FVC and FEV1 agreeing within 100 mL between the best two blows (n=378, 46%).22 Percent-predicted lung function parameters were calculated adopting the European Respiratory Society Global Lung Function Initiative 2012 prediction equations.23
Potential confounders and effect modifiers
Based on previous knowledge, the following variables were considered a priori in the analyses: child’ sex, child's age, height and weight at assessment, child's ethnic background (white children vs other children), birth weight, preterm delivery (<37 weeks of gestation), older siblings at birth, day-care attendance during the first year of life, maternal age at birth, parity (0 vs 1 or more), maternal educational level (primary or less, secondary, university degree), maternal social class (occupation during pregnancy based on the highest social class by using a widely used Spanish adaptation of the international ISCO88 coding system) (I–II, managers/technicians; III, skilled; IV–V, semiskilled/unskilled), maternal prepregnancy Body Mass Index based on height and prepregnancy self-reported weight (kilograms per square metre, kg/m2), maternal and paternal smoking in pregnancy (yes vs no), postnatal environmental tobacco smoke (ETS) exposure during the first year of life and during the last 12 months (yes vs no), duration of breastfeeding (0, <16, 16–24 and >24 weeks), type of cooker at home (electric vs gas), pets and dampness at home, and lower respiratory tract infections (LRTI) during the first year of life.
Child's sex, child and parental allergic history, and current wheezing and asthma at the time of lung function assessment were evaluated as potential effect modifiers. Children and parents were considered as allergic if they reported to suffer from allergic asthma, atopic dermatitis, eczema or allergic rhinitis. We classified children as having current wheezing based on a positive answer to ‘Has ever your child experienced whistling or wheezing from the chest, but not noisy breathing from the nose in the last 12 months?’ Current asthma at the time of lung assessment was defined as a positive answer to either to ‘Has a doctor ever diagnosed your child with asthma?’ or ‘Has ever your child used medication for wheezing during the last 12 months?’
Statistical analysis
Linearity of dose-response relationship between levels of air pollutants and lung function parameters was assessed using adjusted generalised additive models by graphical examination and likelihood ratio testing. Separate multiple linear regression models were run to estimate the associations between levels of residential air pollutants during each specific trimester of pregnancy, early postnatal (during the first year of life), and recent and current exposures with lung function parameters (ie, FVC, FEV1, PEF and FEF25–75) at age 4.5 years. Base models were adjusted for area of study, child's sex, and child’s age, height and weight at assessment, and ethnic background. Fully adjusted models further included all variables that had at least marginally significant association with air pollutant levels (p<0.1) or modified the coefficient of air pollutant levels by at least 5%. Moreover, multiple log-binomial regressions were conducted to estimate associations between levels of air pollutants and clinically low lung function, defined as FEV1 <80% of the predicted value. The measures of associations are presented as the mean difference (mL) in each lung function parameter (linear regression) or the relative risk (RR) for clinically low FEV1 (log-binomial regression), with 95% CIs, for an IQR increase (difference between 25th and 75th percentile) in exposure, to be able to compare the effect of pollutants on lung function. We also estimated lung function changes for a given increase in exposure (1 µg/m³ for benzene and 10 µg/m³ for NO2). Analyses were conducted by using Stata software, V.12.0 (Stata-Corp, College Station, Texas, USA).
Results
From the 1295 women enrolled in the study at the beginning of pregnancy, we obtained data on exposure to both air pollution and lung function assessment at 4.5 years for 620 (48%) of their children (see online supplementary figure S1). Descriptive statistics of the study population, and distributions of lung function parameters are presented in tables 1 and 2, respectively. Lung function parameters at age 4.5 years did not differ between areas of study (all p values >0.35). Compared with excluded participants, mothers of those who were included in the present analysis were older and had higher social class and education level, and children showed higher day-care attendance in the first year of life and higher prevalence of LRTI and wheezing in infancy, but did not differ in other main baseline characteristics (see online supplementary table S2).
Description of study population characteristics
Descriptive lung function parameters in preschoolers aged 4.5 years (n=967)
Table 3 shows the distributions of intrauterine and postnatal exposure to residential air pollutants. Prenatal and postnatal levels of NO2 and NOx were higher in the predominantly urban Sabadell area than in the Gipuzkoa area (see online supplementary table S3). Levels of each pollutant were moderately to highly correlated between trimesters of pregnancy (Pearson coefficients 0.73–0.82), and highly correlated between the entire prenatal period and the first year of life (Pearson coefficient=0.84 for benzene and 0.93 for NO2) (see online supplementary ables S4 and S5). Benzene and NO2 were moderately correlated (Pearson coefficients 0.25–0.55).
Distribution of estimated residential outdoor air pollutants
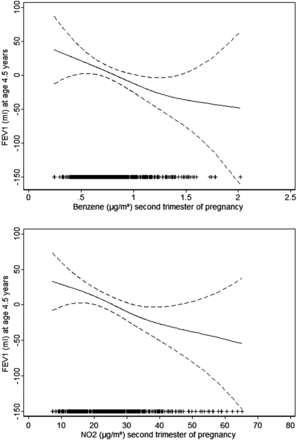
A linear inverse relationship was found between residential levels of benzene and NO2 during pregnancy and parameters in spirometry at age 4.5 years (figure 1). Exposure to higher levels of benzene and NO2 in pregnancy was associated with reduced lung function parameters in spirometry (table 4). FEV1 estimates for an IQR increase in exposure during the second trimester of pregnancy were −18.4 mL, 95% CI −34.8 to −2.1 for benzene; and −28.0 mL, 95% CI −52.9 to −3.2 for NO2. Similar estimates were found using temporally unadjusted air pollutant levels, although statistical significance was weaker (see online supplementary table S6). Estimates for benzene were similar between areas of study, while estimates for NO2 were stronger in Gipuzkoa than in Sabadell area (see online supplementary table S7). Although levels of air pollutants during the first year of life were inversely associated with parameters in spirometry at age 4.5 years the estimates were slightly weaker and not statistically significant (table 4). A 1 µg/m3 increase in benzene and a 10 µg/m3 increase in NO2 exposure during pregnancy were associated with significant deficits in FEV1 at age 4.5 years (estimates for exposures during the second trimester were −51.9 mL, 95% CI −97.9 to −5.9 for benzene; and −17.4 mL, 95% CI −32.8 to −2.0 for NO2) (see online supplementary table S8). Deficits in average lung function associated with higher levels of exposure to benzene and NO2 in pregnancy translated into deficits in percent-predicted lung function estimates. An IQR increase in benzene and NO2 exposure during the second trimester of pregnancy were associated with a decrease in the percent-predicted FEV1 by 1.6% (95% CI −3.2 to 0.0) and 2.7% (95% CI −5.1 to −0.3), respectively (see online supplementary table S8). Recent and current exposures to residential air pollution levels of NO2 and NOx were not associated with significant deficits in lung function (table 5).
Associations of lung function parameters in preschoolers aged 4.5 years with exposure levels of air pollutants in utero and early postnatal (during the first year of life)
Associations of lung function parameters in preschoolers aged 4.5 years with recent and current residential exposure levels of air pollutants
The relation (and 95% confidence levels) of air pollutant levels during the second trimester of pregnancy with FEV1 in preschoolers aged 4.5 years. General additive models adjusted for area of study, child's sex, and child's age, height and weight at assessment and ethnic background, birth weight, maternal social class, maternal education level, maternal smoking in pregnancy and paternal smoking in pregnancy, environmental tobacco smoke 0–14 months, and lower respiratory tract infections 0–14 months. The symbols (+) on the X-axis indicate air pollutant observations.
Moreover, risk of clinically low lung function (<80% of predicted FEV1) increased with exposure to higher levels of benzene and NO2 during pregnancy. RR of low lung function for an IQR increase in benzene and NO2 during the second trimester were 1.22, 95% CI 1.02 to 1.46 and 1.30, 95% CI 0.97 to 1.76, respectively (table 6).
Risk of low lung function (FEV1 <80% predicted) in preschoolers aged 4.5 years in relation to exposure levels of air pollutants
After restricting the analyses to children with reproducible spirometry manoeuvres, estimates for the associations between levels of air pollutants and lung function were essentially the same, although statistical significance was attenuated (see online supplementary table S10). In stratified analyses, we did not find any evidence of different associations for girls and boys. Associations of levels of NO2 during pregnancy tended to be stronger in girls than in boys, but none of the interaction terms were statistically significant (see online supplementary table S11). No differences of the association between levels of air pollutants and lung function parameters were found according to allergic parental status (see online supplementary table S12) and child's asthmatic status (see online supplementary table S13). However, stronger associations were found among allergic children (see online supplementary table S14). Additionally, estimates were essentially the same after excluding infants whose mothers smoked during pregnancy, preterm deliveries and low birthweight newborns (see online supplementary table S15). Stratification by maternal social class showed stronger associations of air pollutants with FEV1 among children of mothers of lower social class (classes III–V) compared with those of high social class (classes I–II) (see online supplementary table S16). Similarly, estimates were stronger among children of mothers with low education levels (primary or less and secondary) compared with those with high education levels (university) (see online supplementary table S17).
Discussion
In this population-based prospective study, higher levels of residential outdoor air pollutant (ie, benzene and NO2) during pregnancy were associated with clinically significant deficits in lung function at preschool age. Associations were robust after adjusting for a large number of potential confounding factors. Associations between early postnatal life (during the first year of life), recent and current exposures to outdoor air pollutants with lung function at preschool age were not statistically significant.
To our knowledge, this is the first study examining effects on lung function as early as at preschool age, in relation to residential exposure to traffic-related air pollutants through different windows of susceptibility including specific trimesters of pregnancy and postnatal lifetime periods. Both, lung volumes (FVC, FEV1) and flow measures (PEF and FEF25–75), showed deficits in relation to higher levels of air pollutants in pregnancy, with stronger associations for the second trimester. FEV1 is a marker of airway obstruction, and flow measures such as PEF and FEF25–75 are considered markers of small-airway function24 ,25 which is particularly sensitive to oxidant air pollutants including ozone26 ,27 and tobacco smoke.28 The magnitude of deficits here reported seems plausible and similar to those previous studied, and translated into higher risk of clinically defined low lung function. Gauderman et al reported that children aged 10–18 years, and living in the most polluted community, had a growth deficit in FEV1 of approximately 100 mL (∼7% for girls, ∼4% for boys), as compared with those living in the cleanest community (exposure range 4–38/ppb NO2).5 In a more recent analysis, Gauderman et al found that children aged 10 years living within 500 m of a freeway had deficits in 8-year growth of FEV1 (−81 mL, 95% CI −143 to −18) compared with children living at least 1500 m from a freeway.6 Rojas-Martinez et al reported that NO2 and O3 levels were associated with annual growth in FEV1 in schoolchildren of Mexico City. Decreases in annual growth in FEV1 per IQR of exposure ranged from −16 mL for O3 (IQR, 11.3 ppb) in boys to −32 mL for NO2 (IQR, 12.0 ppb) in girls.29 Jedrichowski et al have shown that exposure to higher levels of PM2.5 (>52.6 mg/m3) during pregnancy was associated with reduced FVC (−91 mL) and FEV1 (−87 mL) in preschoolers aged 5 years.14 Despite high correlation between prenatal and postnatal levels of air pollutants, our results suggest that in utero exposures and, more specifically during the second trimester of pregnancy, may be more relevant for long-term adverse consequences for lung function than exposures later in life. Our results are in agreement with a previous study that found deficits in offspring lung function at preschool age in relation to maternal short-term exposure to traffic-related air pollutants (ie, PM2.5) in the second trimester of pregnancy.14 Mechanisms underlying the associations of air pollution exposure in pregnancy with reduced lung function in offspring are unknown. Interestingly, respiratory airways development occurs during the second and third trimesters of pregnancy, and continues until 3 years of age.10 ,11 Thus, it is biologically plausible that harmful conditions acting during this crucial period of lung development might have more relevant long-lasting pathophysiological consequences in the lung.
Sensitivity analyses showed that associations between exposure to outdoor air pollutants and lung function at preschool age were not confounded by maternal smoking during pregnancy, either mediated by preterm delivery or low birth weight. Additionally, we did not find any evidence of differential effects according to the child's sex, asthmatic status and allergic parental status, although stronger estimates appeared in allergic children as previously suggested.30 Additionally, we found stronger deficits of lung function in relation to higher levels of air pollutants among preschoolers from middle and low socioeconomic groups, which suggest that socioeconomic status may act as a potential effect modifier of the harmful effects of air pollution on lung function as previously indicated.31 Although the reasons for these differences are not entirely clear, there are some plausible explanations. Several studies have documented that atopy occurs in close association with bronchial hyper-responsiveness, both in asthma patients and in random population samples, which could act synergistically with traffic-related air pollutants. Lower social class households are more likely to be located in areas of poor air quality and higher traffic exposure, and lower social position may make some groups more susceptible to health threats because of factors related to their disadvantage.
The population-based and prospective design of the study set up as early as the first trimester of pregnancy are main strengths of this study. We investigated the potential effects of exposure to residential air pollution during specific periods of pregnancy, and the first year of life on offspring lung function, to identify susceptible exposure windows early in life. We used temporally adjusted LUR models to estimate individual exposures during specific time periods; despite their spatial accuracy, LUR estimates are still a proxy for personal exposure, which may be influenced by individual time-activity patterns.32 Additionally, a large number of potential confounding mediators, and effect-modified factors were considered in the analyses.
This study has some limitations. Loss of follow-up may be a potential source of bias; compared to excluded participants, mothers of those who were included in the present analysis were older and had higher social class and education levels, and children showed higher day-care attendance in the first year of life and higher prevalence of LRTI and wheezing in infancy, but did not differ in other main baseline characteristics. While these differences may have some impact on the generalisability of results, it should not affect their internal validity. We did not measure particulate matters considered good markers of traffic-related pollution. However, NO2 is a widely used marker of traffic-related air pollution, and benzene levels can reflect industrial activities and are considered as a surrogate for a mixture of predominantly traffic-driven pollutants. Air pollution exposures during pregnancy and first year of life tend to be highly correlated, which limits the interpretation of estimates from mutually adjusted models. By contrast with studies that characterise exposures based on measurements from the nearest fixed monitoring stations,33 our exposure assessment approach emphasised spatial over temporal variation, which may have contributed to the very high correlations between prenatal and early postnatal exposures in our study. A different LUR model was used for the more recent (ESCAPE model) exposures than for the pregnancy and early life exposures (INMA model), which may be difficult for direct comparisons. However, for NO2, the ESCAPE model performed well at the ESCAPE sites in Sabadell (R2=0.69), and ESCAPE and INMA-Sabadell model predictions at INMA-Sabadell cohort addresses were relatively well correlated (R2=0.56).34 Not all participants were able to perform spirometry testing; although preschool children are able to perform these manoeuvres.35 Nevertheless, reproducibility rate was nearly 50% in our study, and estimates were essentially the same among participants with reproducible tests. Lack of information on respiratory infection at the current time of lung function testing could have resulted in some residual confounding. Additionally, we cannot exclude potential residual confounding by unmeasured factors including effects of acute recent temperature and maternal occupation exposure to gas, dust or fumes during pregnancy.
In summary, we found that exposure to higher levels of benzene and NO2 during pregnancy was associated with clinically relevant deficits in lung function at preschool age. Results suggest that exposure to traffic-related air pollutants acting during the prenatal period could adversely impact the developing lung. Public policies to reduce exposure to traffic-related air pollution may avoid harmful effects on lung development and function with substantial public health benefits.
Acknowledgments
The authors would particularly like to thank all the participants for their generous collaboration. A full roster of the INMA Project Investigators can be found at (http://www.proyectoinma.org/presentacion-inma/listado-investigadores/en_listado-investigadores.html). The authors are grateful to Inmaculada Aguilera and Marta Cirach for their assistance in air pollution assessment. The authors are grateful to Silvia Fochs, Anna Sànchez, Maribel López, Nuria Pey, Muriel Ferrer, Haizea Begiristain, Bidatz Sasiain and Ainara Andiarena for their assistance in contacting the families and administering the questionnaires.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
PRESS RELEASE
Files in this Data Supplement:
Footnotes
Contributors JS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. EM, OAdlC, MB, JS contributed to the study conception and design. RG-E, OAdlC, MB, AL, MDMLdD, CZ acquired the data. EM analysed the data. EM drafted the manuscript. All authors discussed and interpreted the results, and revised the paper.
Funding This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176 and CB06/02/0041, FIS-PI06/0867 and FIS-PS09/00090), Spanish Ministry of Health (FIS-PI041436, FIS- PI081151), Generalitat de Catalunya-CIRIT 1999SGR 00241, Department of Health of the Basque Government (2005111093 and 2009111069), and the Provincial Government of Gipuzkoa (DFG06/004 and DFG08/001). The funding organisations and sponsors had no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; the preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Competing interests None.
Ethics approval The study was approved by the ethical committees of the centres involved in the study, and written informed consent was obtained from the parents of all the children.
Provenance and peer review Not commissioned; externally peer reviewed.