Article Text
Abstract
Background Bronchodilator responsiveness is a potential phenotypic characteristic of chronic obstructive pulmonary disease (COPD). We studied whether change in lung function after a bronchodilator is abnormal in COPD, whether stable responder subgroups can be identified, and whether these subgroups experience different clinical outcomes.
Methods 1831 patients with COPD, 285 smoking (SC) and 228 non-smoking (NSC) controls from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort. Spirometric reversibility to 400 μg inhaled salbutamol was assessed on four occasions over 1 year.
Results Forced expiratory volume in 1 s (FEV1) increase after salbutamol was similar in SC (mean 0.14 litres (SD 0.15)) and COPD (0.12 litres (0.15)) and was significantly greater than NSC (0.08 litres (0.14)). Reversibility status varied with repeated testing in parallel with the day-to-day variation in pre-bronchodilator FEV1, which was similar in control subjects and patients with COPD. Absolute FEV1 change decreased by Global initiative for chronic Obstructive Lung Disease (GOLD) stage in patients with COPD (GOLD II, mean 0.16 litres (SD 0.17); III, 0.10 litres (0.13); IV, 0.05 litres (0.08) as did chances of being classified as reversible. CT-defined emphysema was weakly related to the absolute change in FEV1 post salbutamol. Consistently reversible patients (n=227) did not differ in mortality, hospitalisation or exacerbation experience from irreversible patients when allowing for differences in baseline FEV1.
Limitations Reversibility only assessed with salbutamol and defined by FEV1 criteria. The COPD population was older than the control populations.
Conclusions Post-salbutamol FEV1 change is similar in patients with COPD and smoking controls but is influenced by baseline lung function and the presence of emphysema. Bronchodilator reversibility status varies temporally and does not distinguish clinically relevant outcomes, making it an unreliable phenotype.
Clinical trial registration number NCT00292552 (http://ClinicalTrials.gov).
- COPD exacerbations
- pulmonary rehabilitation
- COPD mechanisms
- emphysema
- COPD pharmacology
- cystic fibrosis
- cytokine biology
- exhaled airway markers
- innate immunity
- neutrophil biology
- viral infection
- asthma epidemiology
- COPD epidemiology
- assisted ventilation
- lung physiology
- lung volume reduction surgery
- respiratory muscles
- COPD pathology
- imaging/CT MRI etc
- pulmonary embolism
- alpha1 antitrypsin deficiency
- airway epithelium
- macrophage biology
- tobacco and the lung
- ambulatory oxygen therapy
- long-term oxygen therapy (LTOT)
- short burst oxygen therapy
- sleep apnoea
- chronic obstructive pulmonary disease
- bronchodilator reversibility
- phenotype
Statistics from Altmetric.com
- COPD exacerbations
- pulmonary rehabilitation
- COPD mechanisms
- emphysema
- COPD pharmacology
- cystic fibrosis
- cytokine biology
- exhaled airway markers
- innate immunity
- neutrophil biology
- viral infection
- asthma epidemiology
- COPD epidemiology
- assisted ventilation
- lung physiology
- lung volume reduction surgery
- respiratory muscles
- COPD pathology
- imaging/CT MRI etc
- pulmonary embolism
- alpha1 antitrypsin deficiency
- airway epithelium
- macrophage biology
- tobacco and the lung
- ambulatory oxygen therapy
- long-term oxygen therapy (LTOT)
- short burst oxygen therapy
- sleep apnoea
- chronic obstructive pulmonary disease
- bronchodilator reversibility
- phenotype
Key messages
What is the key question?
Is responsiveness to salbutamol a stable phenotype in chronic obstructive pulmonary disease (COPD) and if so, is it a phenotype that predicts outcome?
What is the bottom line?
Bronchodilator responsiveness in COPD does not represent a reliable or useful clinical phenotype.
Why read on?
Evidence from the well characterised Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort of patients with COPD and comparator patients shows that over 3 years reversibility is an unstable characteristic, even when the threshold definition is increased.
Introduction
A chronic obstructive pulmonary disease (COPD) phenotype is defined as ‘a single or combination of disease attributes that describe differences among individuals with COPD as they relate to clinically meaningful outcomes’.1 COPD is characterised by airflow limitation not fully normalised after an inhaled bronchodilator.2 However, some patients increase their forced expiratory volume in 1 s (FEV1) by >12% and >200 ml of the pre-test value, which guidelines define as ‘reversible’.3 Thus reversibility is a candidate COPD phenotype, and has been used by clinicians as a marker for patients more likely to respond to bronchodilators. This approach has been adopted by some medical regulators and is used to define patient subgroups in treatment trials. Recently, reversibility has been linked to a specific COPD genotype.4
However, concerns remain about using reversibility in this way. Although the normalisation of lung function after a bronchodilator in treatment-naive patients excludes a diagnosis of COPD, we do not know whether smaller changes in lung function that meet the accepted criteria for ‘reversibility’ identify discrete patient subgroups, or relate to clinically meaningful outcomes. The prevalence of ‘reversible’ COPD varies,5–7 reflecting differences in patient selection, bronchodilator(s) used and the presence of emphysema.8 Reversibility status also varies between days in some patients with severe COPD,6 which has led to a proposal for higher thresholds to define reversibility in a more meaningful way. Finally, and most importantly, it is unknown whether post-bronchodilator change in lung function in COPD differs from that in older healthy subjects.
Here we address these questions by comparing the frequency distribution and absolute change in FEV1 post bronchodilator in patients with COPD and in smoker (SC) and non-smoker (NSC) controls; determining the temporal stability of COPD reversibility and factors that contribute to differences between patients; and determining whether consistently reversible patients have different clinical outcomes from those who are not. To do this, we used data from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study, in which bronchodilator reversibility data were collected using the same methodology on multiple occasions in patients with COPD and comparator subjects.
Methods
Design overview
ECLIPSE (NCT00292552; SCO104960) is a 3-year, non-interventional prospective study conducted at 46 centres in 12 countries.9
Setting and participants
ECLIPSE recruited 2164 patients with COPD (40–75 years) with clinically diagnosed COPD, a post-bronchodilator FEV1/forced vital capacity (FVC) ratio ≤0.7 and FEV1 <80% of predicted. All were current/former smokers of ≥10 pack-years and exacerbation free for at least 4 weeks. Three hundred and thirty-seven SC (current/former) and 245 NSC (<1 pack-year) subjects also participated. All subjects gave written informed consent and the study protocol was approved by all relevant research ethics committees. Only subjects with complete data at baseline, 3, 6 and 12 months are included in this analysis.
Outcomes and follow-up
At each visit participants performed spirometry (VIASYS MasterScope) to American Thoracic Society (ATS)/European Respiratory Society (ERS) standards,10 before and 15 min after inhaling 400 μg salbutamol.
At visit 1, a low-dose CT scan was performed to determine emphysema severity. Objective emphysema severity was defined by the percentage of CT voxels with x-ray attenuation values <–950 Hounsfield units (HU) (per cent low attenuation area, %LAA) as described previously.11 Subjective emphysema was assessed by two independent radiologists who scored radiographical emphysema severity.
Further details of spirometric and CT methodology are given in online appendix supplement 1.
Data analysis and statistical methods
Data are presented as mean and SD for continuous variables, and counts/frequencies for categorical variables unless otherwise stated. Only subjects with reversibility data at all four visits are reported here. Response is expressed as absolute volume change or as change in %FEV1 predicted for sex-related differences and relationship to pre-salbutamol FEV1. Reversibility was defined by ATS/ERS criteria of ≥12% and ≥200 ml increase from pre-bronchodilator FEV13 and absolute response of >400 ml Skewness and kurtosis were calculated for each distribution of absolute FEV1 change. Comparisons between subject groups were carried out by analysis of variance and pairwise contrasts or Cochran–Mantel–Haenszel tests, as appropriate. Linear and logistic regression was used to examine factors potentially associated with response measured by absolute change in FEV1 or reversibility (ATS/ERS criteria), respectively. Mean pre-bronchodilator FEV1 of the four attendances for each subject was calculated and we tested whether a positive reversibility response was associated with the subject being above or below this mean lung function value. Finally, selected clinical outcomes (mortality, withdrawal after the year of follow-up, annual hospitalisation rate, and exacerbation frequency calculated as described elsewhere12) were compared via logistic regression and negative binomial regression in patients who met the ATS/ERS reversibility criteria on at least three of four occasions with those who did not. Spearman's ρ was calculated to describe the magnitude of linear correlation between variables. p Values lower than 0.05 were considered significant. No adjustments for multiple comparisons were done. All analyses were conducted with SAS V.9.1.
Results
Demographics and characteristics of the analysis population (COPD=1831, SC=285, NSC=228) did not differ from the full ECLIPSE population.13 (table 1). The use of tiotropium did not affect the likelihood of being classed as reversible in any GOLD stage. Four hundred and two patients reported previous asthma or an asthma diagnosis on the respiratory questionnaire. Their lung function changes in FEV1 after salbutamol were no different compared with patients without this diagnosis (online appendix table 1).
Demographics and background characteristics of participants in the study
Comparison of the bronchodilator response between groups at baseline
At baseline, change in FEV1 post salbutamol was not normally distributed in the three groups studied (online appendix figures 1A–C) or within GOLD stage (online appendix figures 1D–F); measures of skewness and kurtosis suggest positive skewness with higher peaks than might be expected in a normal distribution. FEV1 (mean (SD)) increased more in patients with COPD (0.12 litres (0.15)) and SC (0.14 litres (0.15)) than in NSC (0.08 litres (0.14); p<0.001). FEV1 response differences between COPD and SC were not significant at baseline (p=0.11) or subsequently (tables 2 and 3).
Change in lung function, as measured every 3 months, patients with COPD and the comparator groups
Change in lung function, as measured every 3 months, among patients with COPD according to disease severity (GOLD)
At baseline, 24% of patients with COPD met ATS/ERS reversibility criteria, contrasting with 5% SC and 2% NSC. Using a 400 ml volume change to identify reversibility classified 5% of patients with COPD, 4% SC and 1% NSC as reversible.
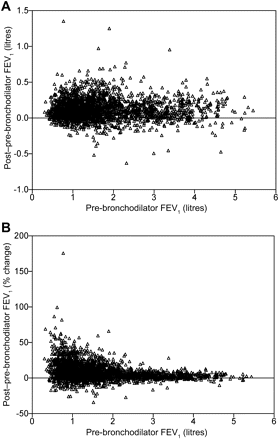
Absolute change in FEV1 post salbutamol was largest in patients with GOLD II and smallest in patients with GOLD IV disease (p<0.001 for all comparisons; tables 2 and 3). It was not related to pre-test FEV1 (figure 1A, r=0.04, p=0.075) but when expressed as a percentage change increased as pre-salbutamol FEV1 declined (figure 1B, r=–0.32, p<0.001). As fewer patients met the volume criterion, FEV1 reversibility was less likely in those with GOLD IV than in those with GOLD II disease (OR 0.129 (95% CI 0.074 to 0.764), p<0.001).
The relationship between the change in forced expiratory volume in 1 s (FEV1) post bronchodilator and the pre-bronchodilator FEV1 expressed as an absolute value (A) or as a percentage change from baseline (B) at visit 1.
In the whole COPD group post-salbutamol change in FVC paralleled FEV1 (tables 2 and 3), leaving the mean FEV1/FVC ratio unchanged. In contrast, a small but significant increase (p<0.001) in the FEV1/FVC ratio in the control groups was driven by increased FEV1 (tables 2 and 3). Between GOLD stages change in FVC was similar, but change in FEV1 declined with increasing severity, producing significantly different changes in the ratio (tables 2 and 3). In GOLD II the FEV1/FVC ratio increased post salbutamol, was unchanged in GOLD III and became negative in GOLD IV due to the small change in FEV1 relative to the increase in FVC, i.e. FVC increased by 0.25 litres from 3.14 litres in GOLD II, and by 0.25 litres from 2.13 litres in GOLD IV (tables 1–3). Between-group differences for change in FEV1 and FEV1/FVC were significant (p≤0.031).
Temporal stability of reversibility status in patients with COPD
In the COPD group, mean post-salbutamol response in FEV1 and FVC were similar at each visit so the total number of reversible subjects was stable between visits. However, there was significant individual between-visit variability in reversibility status. Only 16% of subjects considered reversible at the first visit met the ATS/ERS reversibility criteria at all subsequent visits while 66% considered initially irreversible were irreversible at all visits (figure 2A).
The reproducibility of the classification of reversibility in patients with chronic obstructive pulmonary disease followed on four occasions over 1 year. (A) Using the American Thoracic Society/European Respiratory Society reversibility criteria; (B) change in absolute FEV1 of more than 400 ml.
The use of a larger absolute volume change (400 ml) to define reversibility did not abolish this variability, 11% were reclassified on at least one visit and only 4% of subjects showed reversibility so defined on two or more occasions (figure 2B).
Predictors of reversibility status in COPD
Age, smoking status (see online appendix table 2), and cumulative smoking exposure (pack-years) were not related to post-salbutamol change in FEV1, or to ATS/ERS reversibility status at visit 1. Men showed a greater FEV1 improvement than women (0.3 litres) and had 11% more chance of being classed as reversible. However, because men and women differed in their pre-salbutamol lung function, they showed a similar increase in FEV1 when expressed as per cent predicted (4.3% in each group) (table 4).
Lung function characteristics in patients with COPD and comparator subjects according to sex
The degree of emphysema (quantitative or subjective) generally increased as FEV1 fell (p<0.001; table 1). Objective emphysema (%LAA) showed a significant but very weak relationship to the absolute change in FEV1 post salbutamol (r=–0.09, p<0.001). When separated into four groups of equivalent size according to the radiologist-defined severity (<5%, 5–25%, 25–50%, >50%) patients with more emphysema (>50%) had the least FEV1 improvement (0.15, 0.14, 0.13 and 0.09, respectively; p<0.001).
Pre-salbutamol FEV1 varied between visits to the same extent in COPD and controls (table 5). Between-visit variation in pre-salbutamol FEV1 decreased with increasing GOLD stage. Patients with COPD were significantly more likely to have a positive bronchodilator response on test days when their pre-salbutamol FEV1 was lower than its mean value derived from all four visits (p<0.001).
Consistency of reversibility status and clinical outcomes summary of pre-bronchodilator FEV1 at each visit
Relationship of reversibility status with clinically relevant outcomes
Although patients judged ‘irreversible’ on three of the four occasions tended to a higher mortality and were more likely to be hospitalised and withdraw from follow up these differences were not statistically significant (table 6). When we used logistic regression to compare frequent (≥2/year) with never exacerbators, reversibility status identified frequent exacerbators (unadjusted OR (95% CI) 0.537 (0.377 to 0.764), p<0.001). This association was unaffected by age, sex, smoking status and body mass index in a multivariate model but disappeared when pre-salbutamol FEV1% predicted was included as a covariate (OR 0.901 (95% CI 0.617 to 1.317), p=0.59).
Consistency of reversibility status and clinical outcomes
Discussion
This is the first report of bronchodilator response in patients with COPD and appropriate comparators using the same methodology. The absolute change in post-salbutamol FEV1 was no different in patients with COPD and smoking controls, but was greater in non-smokers. When classified in a binary manner (reversible or not), there was considerable individual between-visit variation which was reduced but not abolished if a higher threshold for response was chosen. This between-visit variation reflected the spontaneous and physiologically normal day-to-day fluctuation in pre-salbutamol FEV1, which was seen in all study subjects. Age, sex and smoking history did not influence the response in patients with COPD. Irreversible (at least three of four occasions) patients had worse lung function and more CT-defined emphysema, but these features did not preclude a bronchodilator response. Finally, in patients with consistent reversibility or irreversibility, no significant difference was observed with respect to clinically important outcomes, such as mortality, severe exacerbations and withdrawal. While exacerbation rates were higher in those exhibiting consistent irreversibility, no association was observed between reversibility status and frequent (at least two) exacerbations. These findings confirm that bronchodilator responsiveness, whether defined by a single assessment as has recently been suggested14 or by repeated testing as shown here, is not a reliable or clinically relevant COPD phenotype, as has been recently suggested.
Interpretation of findings
In our non-smoker controls the mean change in FEV1 post salbutamol was 80 ml, a constant finding over the study. This is similar to a change of 67 ml reported in a population of asymptomatic non-smokers with a mean age approximately 10 years younger than that reported here.15 FEV1 change in the smoker controls was greater and more variable, as expected.16 The greater absolute improvement in FEV1 was largely explained by the lower pre-salbutamol FEV1 %predicted in the smokers, as post-salbutamol changes in smoker and non-smoker controls were similar. This effect may be due to increased inflammation in the airways of smokers.17 Absolute change in FEV1 was generally similar in COPD and smokers but defining patients with COPD by GOLD stage using post-bronchodilator FEV1 data showed that those with the worse baseline function had the smallest FEV1 increase. This difference, although small, fits with the increased small airways fibrosis seen in COPD18 and the greater degree of emphysema we observed across GOLD stages.
The absolute FVC change was similar across GOLD stages, thus the greater FEV1 increase in GOLD II relative to GOLD IV caused an increase in FEV1/FVC ratio for GOLD II while for GOLD IV the proportionately greater increase in FVC compared with FEV1 resulted in airflow obstruction apparently worsening post salbutamol. Similar ‘volume responders’ have been seen after multiple bronchodilator drugs8 and our data confirm earlier predictions that volume response would be associated with more emphysema.19
Change in FEV1 post salbutamol was not normally distributed in patients with COPD, with evidence of a rightward shift in response in some groups. Women appeared less reversible than men, but this reflected their smaller size and lower pre-test FEV1 as the difference disappeared when post-salbutamol change was corrected for predicted lung function. When post-bronchodilator FEV1 change is considered as a dichotomous, rather than continuous variable, problems arise as the threshold for a significant change is close to the between-test reproducibility for FEV1 measurement.20 The components of the ATS/ERS definition of reversibility affected the probability of a positive response differently. A response based on a percentage change from baseline was more likely at lower FEV1 values, but this was more than compensated for by the effect of the volume threshold which was seldom achieved in patients with severe disease tested with just salbutamol. Increasing the response threshold (>400 ml change) as previously proposed7 substantially decreased the numbers of subjects considered reversible, but did not prevent day-to-day variability in reversibility classification nor did these subjects differ in their baseline characteristics from the remaining patients with COPD. Consequently, problems of reversibility classification are unlikely to be resolved by changes to test criteria.
Although the extent of reversibility in any large subgroup was constant, between tests individual classification varied, independent of age and smoking status. The emphysema data on reversibility were conflicting with no important relationship with objective scoring, but stronger evidence of responsiveness with qualitative scoring. This may reflect the lower baseline FEV1 of patients with severe emphysema and how subjective scores account for hyperinflation and distribution of emphysematous spaces, which are not captured in quantitative scores. Pre-salbutamol FEV1 had a more obvious influence on reversibility; spontaneous variation was similar in all groups, suggesting cholinergic receptor mediated variation in airway smooth muscle tone, a major factor explaining this variability,19 was relatively normal in COPD. When analysed by GOLD stage, pre-salbutamol FEV1 varied less in GOLD IV and this may explain why changes in reversibility were less common in these patients, although they still occurred.
Classifying reversibility status at one visit does not predict clinical outcomes.6 ,21 Our data extend these observations to groups defined by relatively consistent responses to testing. Again there was no association with important outcomes like mortality and study withdrawal. The relationship with reversibility and exacerbation rate reflected the influence of a lower baseline FEV1 which was associated with less reversibility and more exacerbations and explained the apparent association between reversibility and exacerbations. This indirect relationship likely explains other studies in which a relationship between reversibility and outcomes has been suggested. In a previous report22 we found that ECLIPSE subjects meeting ATS/ERS reversibility criteria had a faster decline in lung function (17.4 ml/year) which was most evident in patients with GOLD II disease. This may be due to residual confounding by baseline lung function as happened in our exacerbation analysis since baseline lung function was not included in the multivariate analysis of predictive variables. Whether a patient population exists in which larger responses protect against disease progression would require a longer follow up to resolve than the current data provide.
Strengths and limitations
The lung function changes here were smaller than those previously reported in a 4-year interventional study.7 The most likely reason for this was testing with anticholinergic and β agonists at optimised times,7 as both that study and ours employed rigorous data quality assurance methods.23 Some of the variation in pre-testing FEV1 may have been due to variable adherence to treatment, confounding the pretest measurement. However, most patients in COPD studies use their medication regularly24 and similar changes were seen in the placebo limb of the Inhaled Steroids in Obstructive Lung DiseasE (ISOLDE) study.6 By chance, our non-smoking control group contained relatively more women, but this did not affect findings when the populations were separated by sex. Finally, we focused on the conventional FEV1 definition of reversibility and not on FVC change, which has some theoretical attractions. However, the normal distribution of the FVC response suggests this test would not identify especially responsive individuals.
Clinical implications
In this large convenience sample of patients with COPD mean change in post-salbutamol FEV1 resembled that in smoker controls and was unimodally distributed, suggesting patients with undiagnosed asthma are infrequent among those with COPD meeting our entry criteria (clinical diagnosis, reduced FEV1/FVC). Consequently clinical trials recruiting such patients are unlikely to be confounded by a mixed disease group. As reversibility varies with baseline lung function and sex, reported differences in reversibility in clinical studies are more likely to have arisen by chance and the use of different bronchodilator regimes, rather than by selecting a different type of disease. The FEV1 change we saw was similar to both spontaneous overnight changes reported with and without bronchodilators25 and normal values for diurnal FEV1 variation.26 This suggests that airway smooth muscle behaves normally in COPD and any apparently greater responsiveness is a function of normalising for baseline airway calibre. The preservation of FVC response as the disease worsens aligns with the importance of volume, rather than ‘flow’-related change, in explaining treatment effects.27 Together, these data explain why attempts at defining responder subgroups in clinical trials using spirometry have been largely unsuccessful. Reversibility status on one occasion is an unreliable basis on which to make clinical decisions, no additional clinically useful data (beyond that provided by pre-test FEV1) are obtained when testing on multiple occasions. Whether the degree of day-to-day variability of pre-bronchodilator lung function will prove to be a more useful marker for differences in the natural history of COPD, as suggested by Anthonisen and colleagues,5 remains to be tested in more severe disease and this is a goal in the follow-up phase of the ECLIPSE study.
Conclusions
Our results show that the presence of a positive bronchodilator response, however defined, is not a reliable way to define a specific COPD phenotype or direct clinical management.
Acknowledgments
The authors thank the study participants for their willingness to contribute to the study and acknowledge the ECLIPSE steering and scientific committees and investigators (listed below). The ECLIPSE study is funded by GlaxoSmithKline (http://ClinicalTrials.gov identifier: NCT00292552; GlaxoSmithKline study code SCO104960). The authors would also like to thank Drs Nestor Mϋller and Paola Nasute Fauerbach for their radiological expertise with the assessment of emphysema and Tara Candido, Sebastian Cogswell, Heather Davis, Nima Farzaneh, Lukas Holy, Natasha Krowchuk, Helena Lee, Evan Phillips, Nerissa Tai, Anh-Toan Tran, Nghia Tran, Eugene Wang and Tomonori Yokogawa for technical assistance with the CT analysis and data management, and Juerg Tschirren and Greg Gallardo at VIDA Diagnostics for assistance with the Pulmonary Workstation software. Editorial support in the form of suggestions on a draft version of this manuscript, graphical services for figures and preparing this manuscript for submission was provided by Geoff Weller, PhD at Gardiner-Caldwell Communications, Macclesfield, UK. This support was funded by GlaxoSmithKline. ECLIPSE Steering Committee: Per Bakke (Norway), Harvey Coxson (Canada), Courtney Crim (GlaxoSmithKline, USA), Lisa Edwards (GlaxoSmithKline, USA), David Lomas (UK), William MacNee (UK), Edwin Silverman (USA), Ruth Tal-Singer (Co-chair, GlaxoSmithKline, USA), Jørgen Vestbo (Co-chair, Denmark), Julie Yates (GlaxoSmithKline, USA). ECLIPSE Scientific Committee: Alvar Agusti (Spain), Peter Calverley (UK), Bartolome Celli (USA), Courtney Crim (GlaxoSmithKline, USA), Bruce Miller (GlaxoSmithKline, USA), William MacNee (Chair, UK), Stephen Rennard (USA), Ruth Tal-Singer (GlaxoSmithKline, USA), Emiel Wouters (The Netherlands). ECLIPSE Investigators: Bulgaria: Yavor Ivanov, Pleven; Kosta Kostov, Sofia. Canada: Jean Bourbeau, Montreal; Que Mark Fitzgerald, Vancouver; Paul Hernandez, Halifax; Kieran Killian, Hamilton; Robert Levy, Vancouver; Francois Maltais, Montreal; Que; Denis O'Donnell, Kingston. Czech Republic: Jan Krepelka, Praha. Denmark: Jørgen Vestbo, Hvidovre. The Netherlands: Emiel Wouters, Horn-Maastricht. New Zealand: Dean Quinn, Wellington. Norway: Per Bakke, Bergen. Slovenia: Mitja Kosnik, Golnik. Spain: Alvar Agusti, Jaume Sauleda, Palma de Mallorca. Ukraine: Yuri Feschenko, Kiev; Vladamir Gavrisyuk, Kiev; Lyudmila Yashina, Kiev; Nadezhda Monogarova, Donetsk. UK: Peter Calverley, Liverpool; David Lomas, Cambridge; William MacNee, Edinburgh; David Singh, Manchester; Jadwiga Wedzicha, London. USA: Antonio Anzueto, San Antonio, Texas; Sidney Braman, Providence, Rhode Island; Richard Casaburi, Torrance, California; Bart Celli, Boston, Massachusetts; Glenn Giessel, Richmond, Virginia; Mark Gotfried, Phoenix, Arizona; Gary Greenwald, Rancho Mirage, California; Nicola Hanania, Houston, Texas; Don Mahler, Lebanon, New Hampshire; Barry Make, Denver, Colorado; Stephen Rennard, Omaha, New England; Carolyn Rochester, New Haven, Connecticut; Paul Scanlon, Rochester, Minnesota; Dan Schuller, Omaha, New England; Frank Sciurba, Pittsburgh, Pennsylvania; Amir Sharafkhaneh, Houston, Texas; Thomas Siler, St Charles, Missouri, Edwin Silverman, Boston, Massachusetts; Adam Wanner, Miami, Florida; Robert Wise, Baltimore, Maryland; Richard ZuWallack, Hartford, Connecticut.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online Appendix 1
Footnotes
Funding The ECLIPSE study is funded by GlaxoSmithKline (http://ClinicalTrials.gov identifier: NCT00292552; GlaxoSmithKline study code SCO104960). The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study is funded by GlaxoSmithKline.
Competing interests PA has received speaker honoraria and travel assistance to attend scientific conferences from GlaxoSmithKline and Pfizer. AA has received travel assistance from GlaxoSmithKline to attend ECLIPSE study meetings and honoraria for speaking at conferences and participating in advisory boards from Almirall, Astra-Zeneca, Boheringer-Ingelheim, Chiesi, Esteve, GlaxoSmithKline, Medimmune, Novartis, Nycomed, Pfizer, Roche and Procter & Gamble. LDE, RT-S, BEM, CC and JY are full-time employees of GlaxoSmithKline and hold stock or stock options in GlaxoSmithKline. PMAC has received fees for serving on advisory boards for GlaxoSmithKline, AstraZeneca, Nycomed, Novartis and Boehringer Ingelheim, for expert testimony for Forest/Nycomed, and has received speaker fees from GlaxoSmithKline and Nycomed; he has received travel assistance from GlaxoSmithKline to attend ECLIPSE study meetings and from Boehringer Ingelheim to attend a scientific conference. HOC has received an honorarium for serving on the steering committee for the ECLIPSE project for GlaxoSmithKline. In addition, HC was the coinvestigator on two multi-centre studies sponsored by GlaxoSmithKline and has received travel expenses to attend meetings related to the project. HC has three contract service agreements with GlaxoSmithKline to quantify the CT scans in subjects with COPD and a service agreement with Spiration Inc to measure changes in lung volume in subjects with severe emphysema. HC was the coinvestigator (D Sin PI) on a Canadian Institutes of Health—Industry (Wyeth) partnership grant. HC has received a fee for speaking at a conference and related travel expenses from AstraZeneca (Australia). HC was the recipient of a GlaxoSmithKline Clinical Scientist Award (06/2010–07/2011). EKS received an honorarium for a talk on COPD genetics, grant support for two studies of COPD genetics, and consulting fees from GlaxoSmithKline. EKS received honoraria for talks and consulting fees from AstraZeneca. SR has received fees for serving on advisory boards, consulting or honoraria from Almirall, APT Pharma, Aradigm, Argenta, AstraZeneca, Boehringer Ingelheim, Chiesi, Dey, Forest, GlaxoSmithKline, HoffmanLaRoche, MedImmune, Mpex, Novartis, Nycomed, Oriel, Otsuka, Pearl, Pfizer, Pharmaxis, Merck and Talecris. DAL has received grant support, honoraria and consultancy fees from GlaxoSmithKline. JV has received fees for serving on advisory boards for GlaxoSmithKline, AstraZeneca, Nycomed and Boehringer Ingelheim, and has received speaker fees from GlaxoSmithKline, AstraZeneca, Pfizer, Boehringer-Ingelheim, Chiesi, Novartis and Nycomed; he has received travel assistance from GlaxoSmithKline to attend ECLIPSE study meetings; his wife has previously worked in pharmaceutical companies, including GlaxoSmithKline and AstraZeneca. WM has received travel assistance from GlaxoSmithKline to attend ECLIPSE study meetings. BC has received consulting fees from Altana, AstraZeneca, Boehringer-Ingelheim and GlaxoSmithKline; speaking fees from Altana, AstraZeneca, Boehringer-Ingelheim and GlaxoSmithKline; and grant support from Boehringer-Ingelheim and GlaxoSmithKline.
Ethics approval Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Airwaves