Article Text
Abstract
Background Earlier diagnosis of lung cancer is key to reducing mortality. New evidence suggests that smokers have negative attitudes to screening and participation in lung cancer screening trials is poor (<1 in 6 of those eligible). Understanding participation is important since uptake in screening trials is likely to predict uptake in screening programmes. A qualitative study of people accepting and declining participation in the Lung-SEARCH screening trial was conducted. Two questions were addressed: Are the screening methods offered acceptable to patients? Why do some people take part and others decline?
Methods The qualitative study used semi-structured interviews with 60 respondents from three groups: (a) trial participants providing an annual sputum sample; (b) trial participants with a sputum sample showing abnormal cytology and thus undergoing annual CT scanning and bronchoscopy; and (c) those declining trial participation.
Results Most respondents (48/60, 80%) viewed sputum provision, CT scanning and bronchoscopy as largely acceptable. Those declining trial participation described fear of bronchoscopy, inconvenience of travelling to hospitals for screening investigations and perceived themselves as having low susceptibility to lung cancer or being too old to benefit. Patients declining participation discounted their risk from smoking and considered negative family histories and good health to be protective. Four typological behaviours emerged within those declining: ‘too old to be bothered’, ‘worriers’, ‘fatalists’ and ‘avoiders’.
Conclusion Sputum provision, CT scanning and bronchoscopy are largely acceptable to those participating in a screening trial. However, the decision to participate or decline reflects a complex balance of factors including acceptability and convenience of screening methods, risk perception, altruism and self-interest. Improving practical and changing cognitive aspects of participation will be key to improving uptake of lung cancer screening.
- Exercise
- lung cancer
- lung cancer chemotherapy
- non-small cell lung cancer
- small cell lung cancer
- asthma
- asthma guidelines
- asthma in primary care
- COPD mechanisms
- COPD epidemiology
- COPD exacerbations
- COPD pathology
- tuberculosis
- qualitative
- screening
Statistics from Altmetric.com
- Exercise
- lung cancer
- lung cancer chemotherapy
- non-small cell lung cancer
- small cell lung cancer
- asthma
- asthma guidelines
- asthma in primary care
- COPD mechanisms
- COPD epidemiology
- COPD exacerbations
- COPD pathology
- tuberculosis
- qualitative
- screening
Key messages
What is the key question?
What influences the decision to take part in a lung cancer screening programme?
What is the bottom line?
The decision to participate or decline reflects a complex balance of factors including acceptability and convenience of screening methods, risk perception, altruism and self-interest.
Why read on?
Improving practical and changing cognitive aspects of participation will be key to improving uptake of lung cancer screening.
Introduction
Lung cancer caused 22% of cancer deaths in the UK in 2007, more than double each of the next most common causes (colorectal 10% and breast 8%).1 Lung cancer has a poor prognosis largely because 75% of patients present at a late and incurable stage.2 There is intensive interest in the potential for population screening to improve detection and reduce mortality.3–6 The National Lung Screening Trial in the USA recently reported a 20% fall in lung cancer deaths in screened participants, and results of other large ongoing randomised trials are eagerly awaited.7 However, an important aspect of the cancer screening debate is uptake8—that is, the percentage of subjects invited who actually have the screening test. Low uptake would render a national screening programme non-viable. A relatively high uptake is essential for the success of a national screening programme,9 but this has rarely been addressed in lung cancer screening trials. Of published trials, only one gave sufficient data to calculate a figure (16%),10 with 6% (1 in 17) joining the ongoing Lung-SEARCH screening trial (A Hackshaw, personal communication). Participation in trials of screening may predict uptake in an implemented programme.
Uptake rates of 81%, 80% and 61% are recorded for current UK screening programmes for breast, cervical and colorectal cancer.9 However, lung cancer screening differs from these other cancers because it targets a higher risk subgroup of the population defined by lifestyle, usually smokers and ex-smokers. Emerging data suggest that smokers view screening nihilistically, perceiving early detection and intervention to be of limited use, and being less likely to consider screening than never-smokers.11 The literature on participation in lung cancer screening trials is sparse and limited to a single questionnaire study addressing the NELSON screening trial.12 In those who declined screening, the main reasons were that: participation was too much effort, they lacked understanding of the purpose of the tests and lacked respiratory complaints.12
Lung-SEARCH is a multicentre randomised controlled trial to determine whether screening (annual sputum cytology/cytometry and, if positive, annual CT scanning and fluorescence bronchoscopy) of smokers and ex-smokers with mild or moderate chronic obstructive pulmonary disease (COPD) can identify patients who develop lung cancer at an earlier stage compared with the unscreened group.13 We sought to understand reasons for participation and non-participation to provide knowledge that can be used to improve participation in future trials and a national screening programme.
Questionnaire surveys provide some understanding of rationales for taking part or declining screening and the acceptability of the methods of screening. Semi-structured interviews potentially allow a deeper exploration of beliefs and attitudes. We therefore completed a qualitative interview study exploring acceptability of the methods of screening and reasons for participation and non-participation in the Lung-SEARCH trial. We wished to answer two questions: (1) Are the screening methods (annual sputum cytology, with the possibility of leading to annual CT and fluorescence bronchoscopy) in the Lung-SEARCH trial acceptable? (2) Why do some people decide to take part in the trial and others decline?
Methods
Sampling
Purposive sampling14 was used to identify the three groups of respondents:
Trial participants giving an annual sputum sample.
Trial participants who had produced a sputum sample with cytology showing dysplastic cells and who then underwent annual annual bronchoscopy and CT scanning.
Those who declined taking part in the trial.
Recruitment
Patients are recruited into the Lung-SEARCH trial from both primary and secondary care using searches of GP records and outpatient attendance lists. To be eligible they must be current or ex-smokers with ≥20 pack years and/or ≥20 years of smoking (ex-smokers should have quit within 8 years), have mild to moderate COPD according to the GOLD criteria, a life expectancy ≥5 years and have no serious comorbid conditions.
The following three groups of patients were approached and invited to participate in the qualitative study by their Lung-SEARCH nurse:
Group a: those who had already agreed to participate and had given a sputum sample.
Group b: those who had had a sputum-positive result and so had undergone bronchoscopy and a CT scan.
Group c: those who declined their invitation to participate in the trial. They opted into the qualitative study of participation via a clause in the consent form which allowed us to approach them (if so indicated on the form).
Data collection
Interviews were conducted face-to-face in people's homes where possible, otherwise by telephone. In-depth interviews were used to collect detailed personal information on people's attitudes and beliefs.14 15 We continued recruiting until no new themes emerged from the interviews. Using pilot work and published literature, we developed a topic guide to steer interviews. All interviews were digitally recorded and fully transcribed. Emerging themes influenced the topic guide for future interviews.16
The data were anonymised by removing all identifiable information and allocating an α-numeric code to transcripts.
Data handling and analysis
The framework approach was used to carry out a thematic analysis.17 This is widely used for applied or policy research. Although the process begins deductively with preset aims and objectives, there is an inductive ‘grounded’ reflection of the textual data. We used an iterative process where data analysis influenced further data collection, allowing emergent themes to be explored in future interviews. In addition we gave attention to negative cases contradicting the emerging findings.15–18 The steps of the framework approach are familiarisation, developing a thematic framework, indexing, charting, mapping and interpretation.17 The software package MAX-QDA was used to aid data handling and Excel spreadsheets were used for charting.19 Transcripts were coded independently by two researchers (DP and AB). To test inter-rater reliability we calculated Mezzich's K statistic, giving K=0.75 which indicates substantial agreement.20 To enhance validity we fed findings back to a group of respondents to determine if they agreed with conclusions.
Results
Overview
A total of 60 interviews were conducted among the three groups, 22 face-to-face and 38 telephone interviews (box 1). Over 300 pages of transcripts were produced, subsequently generating nine categories with 48 sub-categories. These were refined to produce four main themes (box 2).
Numbers and types of interviews
Group a: 16 respondents in the LUNG-Search trial giving an annual sputum sample (9 face-to-face and 7 telephone interviews).
Group b: 20 respondents in the trial receiving annual bronchoscopy and CT scanning (5 face-to-face and 15 telephone interviews).
Group c: 24 respondents who declined to take part in the trial (10 face-to-face and 14 telephone interviews).
Main themes
Acceptability of the screening methods
Taking part
Perceptions of risk
Barriers to participation
The demographics of the three groups of respondents in the study are given in table 1.
Summary of the characteristics of the three interviewed samples
Acceptability of screening methods
Screening methods (sputum cytology, CT scanning and bronchoscopy) in the Lung-SEARCH trial were broadly acceptable to the majority of respondents (48/60; 80%). The exception was that bronchoscopy was perceived adversely by many (18/24; 75%) who declined participation in the trial.
Providing sputum samples
Most had no concerns about giving an annual sputum sample. Although two trial participants mentioned they were ‘not able to get phlegm’, it was a minor concern and did not discourage participation. One respondent who declined participation considered producing sputum disgusting and an important deterrent (box 3).
Providing sputum samples

Views of bronchoscopy
Most, but not all, participants who had undergone bronchoscopy (Group b) considered it largely acceptable. Although some reported it ‘a little bit unpleasant’, this had not deterred them from having future bronchoscopies. One respondent, however, described a particularly distressing experience of bronchoscopy (box 4). Even those in the sputum only group who had not experienced bronchoscopy thought it worth the discomfort ‘if it was to be done on something suspicious’. Indeed, bronchoscopy was ‘having a good look inside me’ (box 4).
Views of bronchoscopy (trial participants)

Respondents declining trial participation (Group c) perceived bronchoscopy adversely (box 5). Most had heard of or experienced negative aspects. For some the mere description of the test was ‘disgusting’.
Views of bronchoscopy (trial decliners)

Experiences and perceptions of CT scans
The majority of respondents (59/60; 98%) across the three groups gave positive views about CT scans; even those who declined participation had no concerns (box 6).
Experiences and perceptions of CT scans

Motivations to take part
Key motivations for taking part included the possibility of early detection of lung cancer, the reassurance provided by negative test results, altruism, having known others with lung cancer and accurate risk perception (box 7).
Motivations to take part in a screening trial

Altruism
Most (4/7) of the older participants (age >70) offered altruistic reasons for agreeing to participate compared with younger participants. Altruism related to improving the research ‘for those doing the trial to learn’, for younger relatives, ‘I mean I've got grandchildren, so if something happens to them’, and ‘for people in general’. However, none agreed to participate purely for altruistic reasons; those who wished to act for the greater good also gave reasons representing self-interest (box 7).
Personal benefit
All participants who agreed to take part in the trial expressed views on the benefits of the trial for themselves, irrespective of age, gender or smoking status. By ‘having a good look inside me’, early detection of cancer was the main hoped-for personal benefit of participation in screening.
A few respondents who declined to take part in the trial also reported ‘early detection’ as a benefit of screening; however, their desire not to participate outweighed any perceived personal benefits of screening, perhaps because they considered themselves at low risk of cancer.
Reassurance
Participants also indicated that participation provided reassurance. This was expressed as a sense of security or relief, even ‘feeling wonderful’, particularly after a negative screening result. The idea that someone ‘was keeping an eye’ on them was a comfort that was appreciated. Acknowledging that spouses also worried about lung cancer encouraged some to participate.
Knowing other people with lung cancer
Having known friends or relatives with or dying of lung cancer led to a strong desire to prevent a similar fate for themselves. Perceived risk of lung cancer was highest in these participants.
Perception of risk of lung cancer
Perception of risk of lung cancer played a key role in the decision to take part or not. Over three-quarters of those taking part in the trial judged themselves to be at significant risk (28/36; 78%) which contributed to their decision. By contrast, those who declined appeared to underestimate or deny their risk. Reasons given by decliners included a lack of current or past lung complaints, adequate current care or a negative family history. Those who considered themselves at risk attributed this to many years of smoking and positive family histories of lung cancer.
Influence of family history on risk
Family history was raised as a key determinant of risk of lung cancer by those taking part and those declining participation. Some decliners perceived a negative family history as an indication that they were protected against the effects of their continued smoking.
Influence of current health and medical care on risk
Some respondents considered their risk of lung cancer in relation to their current health status, with absence of symptoms interpreted as indicating a low risk of cancer. Others felt that they were receiving adequate care for their COPD and were therefore not at risk.
Barriers to participation
Travelling for screening tests
The need to travel to study centres for CT scans and bronchoscopy in the event of positive sputum cytology was an important factor in the decision for those who declined participation. Half of the respondents said the possibility of travel was their most significant reason to decline. Several of the respondents said they would join the trial if any possible tests could be performed at their local hospital (box 8).
Barriers to participation

Bad experiences of hospitals and doctors
Inconvenience of cancelled appointments and ‘very bad experiences’ of hospitals and doctors led some to decide that involvement in the trial would have been an unnecessary burden.
Perception of bronchoscopy
As already discussed, negative perceptions of bronchoscopy were a powerful deterrent to participation.
Reaching a decision about participation
Reaching a decision about participation often involved weighing multiple factors for and against. Spouses sometimes contributed to the decision process (box 9).
Deciding whether to take part

Typologies of patients declining participation in screening
Four attitudinal or behavioural typologies emerged from the analysis of those who declined the trial: (1) Many considered themselves ‘too old to be bothered’ or ‘too old to benefit’. (2) Worriers comprised female respondents who said participating in screening would only increase their anxieties. (3) Fatalists were all current smokers who believed in the inevitability or predetermination of events and reported that ‘if they were going to get it (lung cancer), then they would get it’ and taking part in screening was not going to change that. (4) Avoiders would rather not know if they had lung cancer (box 10). See online appendix for full list of respondents' quotes.
Typologies of patients declining participation in screening

Discussion
Summary of key findings
Our study is the first using qualitative methods to examine attitudes to participation in a lung cancer screening trial, and only the second to address this topic.12 Our findings complement and extend those from previous quantitative work.12
Screening methods—sputum provision, CT scanning and bronchoscopy—were largely acceptable to participants in the Lung-SEARCH trial. For some who declined participation, fear of bronchoscopy was an important factor. Patients balanced a range of factors when reaching a decision about participation in the screening trial. These included their knowledge of screening tests, perceived of risk of lung cancer, family history of cancer, senses of altruism and self-interest, potential to benefit in relation to their age, their current health, experiences of doctors and hospital care and convenience/practicalities of travelling for hospital tests. Those declining participation emphasised unacceptability and inconvenience of tests, underestimated their personal risk and, notably for older patients, felt that the benefit of early detection of cancer was negligible in the face of advanced years. Reluctance among elderly people to be screened is important since half of all lung cancers occur in patients aged >70 years.21
Self-interest was relevant to both those accepting and declining participation—for the former, the potential for early detection and the sense that they were being monitored for cancer were key, for the latter, self-interest reflected a desire to avoid unpleasant tests, inconvenience and discomfort, and raised anxiety. The potential to raise anxiety may be especially marked when screening for lung cancer, where patients are often acutely aware of their lifestyle risks. Breast and colon screening studies suggest that ‘cancer-related worry is often associated with perceived risk’.22 In the literature on cancer screening there is debate as to whether worry leads to or deters people from cancer screening.23–25 However, our findings suggest that anxiety deterred participation in screening.
While altruism was largely expressed by participants as the desire to help other people by taking part in research, it was also seen as helping relatives by reassuring them that the participant did not have lung cancer. Those declining participation did not discuss altruism, perhaps because it would reflect them in a bad light. Although previous work has highlighted the importance of altruism in decisions concerning trial participation,26 our study shows that altruism was often accompanied by self-interested motives. Altruism is perhaps the only factor we explored that did not relate to participation in a future screening programme.
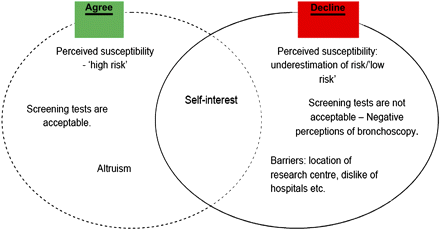
Figure 1 illustrates these factors and the ways in which they overlap in patients' deliberations and decisions.
Reasons for declining or agreeing to participate in the Lung-SEARCH screening trial.
Strengths and weaknesses of the study
Qualitative methods are well suited to exploring attitudes and beliefs. A constant comparison approach allowed us to explore emerging themes such as age and perceived benefit of screening. We achieved data saturation and interviewed a large number of people who declined participation in the trial, who are often hard to reach. We enhanced the validity of our findings through respondent validation—feeding findings back to a group of respondents to determine if they agreed with the conclusions. Our findings triangulated well with those of the questionnaire study of participation in the NELSON trial.12 Reliability was ensured in several ways. The interviews were digitally recorded and professionally transcribed, thus eliminating potential bias through note-taking and researcher transcription. The framework of codes was developed by group discussion and inter-rater reliability was achieved through comparison of individually coded transcripts. MAX-QDA software was used to allow systematic searches through the data to retrieve relevant sections.
Weaknesses of the study include a limited number of respondents in work (most were retired) and limited numbers of ethnic minority patients. Although our figures are representative of the trial population, this may limit generalisability of our findings.
Comparison with other data
Our findings echo and extend those of Bergh et al who used a questionnaire survey to examine participation in the NELSON screening trial.12 The most common reason for participation in the NELSON trial was the possibility of early detection of lung cancer, followed by heavy smoking, desire for reassurance and altruism. For those who declined participation, the most common reasons were ‘too much effort’, followed by lack of understanding of the purpose of the tests, lack of lung complaints and anxiety about lung cancer.12
Patients who undergo bronchoscopy as an investigation (rather than a screening tool) report moderate to high satisfaction, with 71% and 98% of patients saying they would definitely return for a bronchoscopy.27 28 This is consistent with our findings.
While spiral CT scanning may prove to be the optimal primary screening test rather than sputum cytology,11 both tests were largely acceptable to our respondents. CT scanning requires travel to a central facility which might affect uptake slightly in a screening programme.
Questionnaire studies have found that smokers and ex-smokers underestimate their risk of lung cancer,11 12 and our findings reiterate this. Bergh et al12 also reported that those declining screening underestimated the risk of cancer compared with those accepting screening.
Echoing the findings of Silvestri et al,11 we found that ex-smokers predominated over smokers in the groups we interviewed who accepting screening and vice versa for those declining (table 1), suggesting a negative attitude among smokers towards screening.
Implications of our findings
Our findings have implications for the conduct of screening programmes and trials. For research, further work could explore how people perceive risk and how this is related to ageing, and the role of fatalism in the health behaviour of smokers. For screening programmes and trials, if bronchoscopy is found to be useful as part of a screening programme, more work needs to be done to present it as an acceptable screening tool. In addition, attempts to maximise participation in lung cancer screening programmes should recognise that decisions to take part involve complex judgements by patients. These judgements reflect practical issues (such as getting to hospital, acceptability of bronchoscopy) and cognitive (risk/benefit) judgements, the latter often affected by personal traits and experience. Tools to make explicit to patients their risk of cancer may be only partially effective without parallel interventions to address these other factors.
Acknowledgments
We thank all participants, members of the Lung-SEARCH teams who helped identify patients for participation and Cancer Research UK for funding the Lung-SEARCH screening trial.
Appendix
Lung-SEARCH investigators
Dr Paul Plant, Respiratory Medicine, St James's University Hospital, Leeds, UK
Dr Mick Peake, Department of Respiratory Medicine, University Hospitals of Leicester, Glenfield Hospital, Leicester, UK
Dr Robert Rintoul, Department of Thoracic Oncology, Papworth Hospital, Cambridge, UK
Dr Jeremy George and Dr Sam Janes, Department of Thoracic Medicine, University College London Hospitals, London, UK
Dr Pallav Shah, Royal Brompton Hospital and Chelsea & Westminster Hospital, London, UK
Dr Niall Keaney, City Hospitals Sunderland NHS Foundation Trust, Chest Clinic, Sunderland Royal Hospital, Sunderland, UK
Dr Paul Dhillon, University Hospitals Coventry and Warwickshire NHS Trust, University Hospital, Coventry, UK
Dr Nick Magee, Respiratory Research Office, Belfast City Hospital, Belfast, UK
Dr Richard Booton, North West Lung Centre, Wythenshawe Hospital, Manchester, UK
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data Supplement - Manuscript file of format pdf
Footnotes
Linked article 200898.
DP and AA contributed equally to this work.
↵* The Lung-SEARCH Investigators are listed in appendix 1.
Funding Cancer Research UK (CRUK) 2225.
Competing interests None.
Patient consent Obtained.
Ethics approval Ethics approval was provided by South West London Multi Research Ethics Committee.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Our primary data is available for examination on request.