Article Text
Abstract
Patients with severe refractory asthma pose a major healthcare problem. Over the last decade it has become increasingly clear that, for the development of new targeted therapies, there is an urgent need for further characterisation and classification of these patients. The Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes (U-BIOPRED) consortium is a pan-European public-private collaboration funded by the European Commission Innovative Medicines Initiative of the European Union. U-BIOPRED aims to subphenotype patients with severe refractory asthma by using an innovative systems biology approach. This paper presents the U-BIOPRED international consensus on the definition and diagnosis of severe asthma, aligning the latest concepts in adults as well as in children. The consensus is based on existing recommendations up to 2010 and will be used for the selection of patients for the upcoming U-BIOPRED study. It includes the differentiation between ‘problematic’, ‘difficult’ and ‘severe refractory’ asthma, and provides a systematic algorithmic approach to the evaluation of patients presenting with chronic severe asthma symptoms for use in clinical research and specialised care.
- Asthma
Statistics from Altmetric.com
Introduction
Since the last international consensus meetings in 1999 (European Respiratory Society (ERS) Task Force1) and 2000 (American Thoracic Society (ATS) Workshop2), novel insights into an accurate definition of severe asthma have emerged. Defining and phenotyping of patients with severe asthma is becoming increasingly important for the development of new therapies,3–6 as some patients who were previously classified as having severe asthma because of poor asthma control despite maximal doses of conventional therapy may eventually become well controlled with a targeted phenotype-specific treatment.7–14 In addition, it seems appropriate to differentiate between ‘difficult-to-treat’ and ‘severe refractory’ asthma, since these subtypes of asthma may all present with severe symptoms but could represent different conditions or phenotypes with disparate underlying causes that would benefit from targeted therapy.
Diagnosis and definition of severe asthma over the last 15 years
Various documents proposing different clinical definitions of ‘severe asthma’ in adults and children have been published over the last 15 years by international task forces, workshops, networks and guideline committees.
Adult guidelines
In the Global Initiative for Asthma15 1995 and 2002 updates and the National Asthma Education and Prevention Programme 199716 guidelines, overall asthma severity was primarily based on the patient's clinical characteristics prior to commencing treatment. Off-treatment severity was classified into intermittent, mild persistent, moderate persistent and severe persistent, based on symptoms, short-acting β2 agonist use, night time awakening and peak expiratory flow or the percentage predicted forced expiratory volume in 1 s (FEV1). This initial classification was used to determine the patient's initial treatment but did not take into account disease responsiveness to treatment.
In 1999 an ERS Task Force1 defined ‘difficult/therapy-resistant asthma’ as poorly controlled asthma and a continued requirement for short-acting β2 agonists despite delivery of a reasonable dose of inhaled corticosteroids (ICS) and follow-up by a respiratory specialist for a period of >6 months. During this period, asthma management had to be carried out according to published asthma guidelines.
In 2000 an ATS Workshop2 adopted the term ‘refractory asthma’ and developed a definition by consensus. The definition included one of two major criteria (continuous high-dose ICS or oral corticosteroids for >50% of the time during the previous year), with two out of seven additional minor criteria: requirement of additional controller medications, aspects of disease stability, exacerbations and lung function. This definition was adopted by the NIH/NHLBI-sponsored Severe Asthma Research Program (SARP)17 network.
The European Network for Understanding Mechanisms of Severe Asthma18 defined ‘severe asthma’ in 2003 as confirmed asthma (typical asthma symptoms, reversibility in FEV1 or airway hyper-responsiveness) plus the occurrence of one or more exacerbations in the previous year despite oral corticosteroids or high-dose ICS.18 The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study group19 included patients with high use of the healthcare system or high medication use in the past year.
In 2007 an international workshop was organised in Paris to discuss the important questions in severe asthma.20 This workshop agreed that a diagnosis of ‘severe asthma’ should be reserved for those patients who have refractory asthma after an extensive re-evaluation of the correct diagnosis, aggravating comorbidities and environmental factors and an appropriate observation period of at least 6 months.
Paediatric guidelines
In children there has been a lack of general consensus on the definition of severe asthma. In 2008 an ERS Task Force on Definition, Assessment and Treatment of Wheezing Disorders in Preschool Children21 stated that making a diagnosis of asthma in preschool children is unfeasible. Whereas in adults and children >6 years of age there is consensus that asthma is characterised by airway inflammation,15 this has been poorly studied in preschool children,22 and may be absent in very young children who wheeze.23 The Task Force members therefore adopted a symptoms-only descriptive approach for children <6 years of age, and used the terms ‘episodic (viral) wheeze’ to describe children who wheeze intermittently and are well between episodes and ‘multiple-trigger wheeze’ for children who wheeze both during and outside discrete episodes.21
In 2008 the Problematic Severe Asthma in Childhood Initiative (PSACI) group24 proposed the use of the term ‘problematic severe asthma’ to describe all school-aged children who, despite regular treatment with ≥800 μg/day budesonide or equivalent of ICS plus a long-acting β-agonist, a leukotriene receptor antagonist or theophylline, have poorly controlled asthma—that is, daily asthma symptoms, recurrent severe asthma exacerbations (or a single near-fatal asthma attack), persistent airflow obstruction or the necessity for the prescription of chronic oral steroids to achieve control of asthma. ‘Difficult-to-treat asthma’ was defined as asthma where the poor control is due to a wrong diagnosis or comorbidities, the inability and unwillingness to adhere to the prescribed treatment regimens or adverse psychological and environmental factors. ‘Severe therapy-resistant asthma’ was defined by the same group as ‘difficult’ asthma that remains uncontrolled despite attention to and resolution of all these factors.
Shortcomings of previous definitions on severe asthma
When studying the above-mentioned definitions of ‘severe asthma’, it appears that they have been refined and sharpened over the years. After the initial Global Initiative for Asthma 200515 and National Asthma Education and Prevention Programme 199716 guidelines in which overall asthma severity was based on the patient's clinical characteristics prior to commencing treatment, an assessment of asthma severity in patients on treatment seemed to be necessary.25
The definition of ‘difficult/therapy-resistant asthma’ by the ERS Task Force in 19991 described patients with poorly controlled asthma despite prescription of a reasonable dose of ICS (defined as ≥2000 μg beclometasone or the equivalent dose in adults and ≥800 μg beclometasone or equivalent), and emphasised the need for addressing (1) the diagnosis of asthma; (2) adequate management of asthma; (3) compliance with treatment; (4) identification of exacerbating factors; and (5) exclusion of other diagnoses. Care of the patient by a respiratory specialist for at least 6 months was advisable. The proposed definition of the ERS Task Force was inclusive with the recognition that difficult/therapy-resistant asthma could be due to poor adherence, incorrect inhaler technique, psychological problems and comorbidities. It was also recognised that the definition could be adjusted according to the objectives of any individual research project.
The criteria for ‘refractory asthma’ as determined by the ATS Workshop in 20002 were more strictly defined in terms of the criteria of severity of asthma (≥2 of 7 criteria) in patients who were on high-dose ICS and/or oral corticosteroids for >50% of the time. Moore and colleagues17 in their 2007 report on patients with severe asthma in the Severe Asthma Research Project (SARP) using the definition of the ATS Workshop found that the factors that best discriminated mild/moderate from severe asthma (apart from the use of high-dose ICS or oral corticosteroids >50% of the time) were the use of multiple controller medications (including long-acting β2 agonists), ≥3 bursts of oral corticosteroids in the previous year or a history of at least one severe exacerbation requiring hospitalisation during the last year.
The most recent definitions of severe therapy-resistant asthma are the ones proposed by the Paris Workshop in 2007 for adults,20 and the PSACI group in 2009 for children.26 These definitions distinguished patients with ‘severe refractory asthma’ from those with ‘difficult-to-treat asthma’, the latter presenting with uncontrolled asthma due to other factors than asthma itself, including persistent environmental exposures, aggravating comorbidities, poor adherence and inadequate inhalation technique. This distinction is important because patients with difficult-to-treat asthma may not be candidates for immune suppressive or innovative anti-inflammatory therapies.
Finally, in April 2009 the WHO Consultation on Severe Asthma proposed a global definition of asthma severity which should be applicable in most circumstances in low-, middle- and high-income countries.27 Severe asthma was defined as ‘uncontrolled asthma which can result in risk of frequent severe exacerbations (or death) and/or adverse reactions to medications and/or chronic morbidity (including impaired lung function or reduced lung growth in children)’. The WHO Consultation adopted the definitions of ‘severe’ and ‘difficult’ asthma from the Paris Workshop in 200716 and extended it with a third group of patients with ‘untreated’ severe asthma. The latter group is, of course, of major importance in low-income countries where asthma drugs are not readily available to everyone and asthma deaths are still occurring.
Approach to evaluating patients with severe asthma symptoms
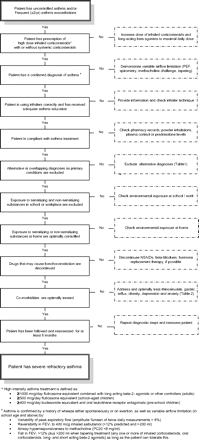
For a correct diagnosis of severe refractory asthma, it is mandatory that patients who present with severe asthma symptoms or recurrent exacerbations are evaluated in a stepwise manner to address the following issues (figure 1).
Algorithm to diagnose a patient with severe refractory asthma. NSAID, non-steroidal anti-inflammatory drug; PEF, peak expiratory flow.
Distinction between severe and uncontrolled asthma
Severe asthma should be distinguished from uncontrolled asthma. Uncontrolled asthma refers to the extent to which the manifestations of asthma have not been reduced or removed by treatment.28 Asthma control incorporates components of current clinical control including symptoms, use of rescue medication and lung function, as well as future risks. Asthma severity is determined by the intensity and phenotype of the underlying disease, both of which may be characterised by pathological and physiological markers. These markers can also be used to estimate future risk of exacerbation or decline in lung function.
Exacerbations are a prominent feature of both poorly controlled and severe asthma. Improving baseline asthma control with ICS can reduce the risk of exacerbations in patients with atopic asthma,29 but control of daily symptoms does not always imply control of exacerbations.30 Baseline disease control and exacerbations are most probably driven by different factors.
Adherence to high-intensity asthma treatment
A significant proportion of patients with uncontrolled asthma who are prescribed high doses of ICS do not take their medicines. In a case series, 50% of patients prescribed oral steroids were found to be non-adherent when assessed by plasma prednisone and cortisol concentrations.31 Also, other studies in adults and children in the USA and the UK showed that overall adherence to ICS was approximately 50%.32 33 Adherence to ICS was significantly and negatively correlated with the number of emergency department visits, the number of fills of an oral steroid and the total days' supply of oral steroid. Eight per cent of patients never filled their ICS prescription.34 Thus, despite persistent symptoms, many patients choose not to take their prescribed treatment, mainly because they perceive it to be unnecessary, too complex, too expensive in some healthcare systems or because they are concerned about potential adverse effects.35 In the investigation of patients presenting with severe asthma, it is therefore critical to check adherence, either by measuring serum cortisol, prednisolone and theophylline levels where appropriate, making home visits or checking lists of prescriptions from pharmacies. The Medication Adherence Rating Scale (MARS), a questionnaire that has been developed to estimate patient adherence with treatment,36 may be a helpful instrument but needs further validation.
Establishing a secure diagnosis of asthma
There are many conditions that may mimic severe refractory asthma, both in children and in adults. Since these conditions do not respond to high-intensity asthma treatment, they may easily be mistaken for severe asthma. A list of common alternative diagnoses and how they should be diagnosed is given in table 1.
Tests to distinguish severe asthma from alternative diagnosis that may mimic asthma
Every patient with asthma who does not seem to respond to high-intensity asthma treatment should undergo objective tests to confirm the diagnosis of asthma. This includes tests to demonstrate variable airflow limitation such as daily peak expiratory flow measurements, reversibility tests with a bronchodilator drug, challenge tests with a bronchoconstricting agent or a steroid-tapering trial. Although there is no definitive diagnostic test for asthma, the repeated failure to demonstrate variable airflow obstruction over time, with treatment or under bronchial provocation tests should seriously call into question the diagnosis of asthma.
Addressing and treating aggravating factors and comorbidities
Mild to moderate asthma can become severe by the influence of exogenous or endogenous aggravating factors.37 These factors can be either trigger factors, coexisting conditions or part of the asthma syndrome itself.
Trigger factors
Trigger factors include (often concealed) indoor allergens, environmental pollutants, toxic fumes, occupational agents or drugs that can provoke asthma attacks. Several studies have shown that adults and children with severe allergic asthma are exposed to higher levels of allergens at home38 or at school39 to which they are sensitised, compared with subjects with mild asthma. Many patients with severe asthma are sensitised to fungi such as Aspergillus spp., Cladosporium spp., Alternaria spp., Penicillium spp., Candida spp., Trichophyton spp. and others. In these patients, antifungal therapy may lead to significant clinical improvement.40 Unfortunately there is no consensus yet on how to identify relevant environmental triggers.
Patients with asthma who smoke have more severe symptoms, reduced sensitivity to ICS41 and are more likely to be admitted to hospital for asthma than non-smokers with asthma.42 Smoking cessation improves asthma control,43 although complete recovery of steroid sensitivity does not occur in most patients.44 For children whose asthma is triggered by smoke exposure from their parents,45 effective treatment of the child's asthma must include smoking cessation for the child's parents.
Many occupational agents may aggravate asthma in adults. More than 200 high and low molecular agents have been identified that can induce work-related asthma or aggravate pre-existing mild to moderate asthma.46 47
In some patients, asthma severity may be related to specific drugs such as β blockers, ACE inhibitors or aspirin. Non-selective β-blockers48 and ACE inhibitors49 can induce significant bronchoconstriction in patients with asthma after acute dosing. Asthma that is exacerbated by aspirin is usually severe and, in half the patients, adequate control of asthma can only be achieved with oral corticosteroids.50 Removal of all of these drugs may substantially ameliorate asthma, although this is not always the case with aspirin-induced asthma. In this case, aspirin desensitisation might be useful.51
Coexisting conditions (comorbidities)
Disorders that often coexist with severe asthma and may make asthma worse include reflux disease52 and obesity (table 2).53 The exact relationship between gastro-oesophageal reflux and severe asthma has not been fully established, but many studies suggest that there is an interaction in the pathophysiology between the two disease processes. A correlation between gastro-oesophageal reflux and worsening of respiratory symptoms in patients with severe asthma has been convincingly shown,54 but the benefits of antireflux therapy are disappointing.52 It has been suggested that, in this subset of patients, fundoplication may be efficacious.55
Diagnosis and treatment of recognised comorbidities in severe asthma
Asthma and obesity are frequently associated, but the contribution of obesity to ‘difficult-to-treat’ asthma and the mechanisms responsible for this relationship have not been fully clarified. Bariatric surgery may lead to substantial weight loss and is associated with substantially decreased asthma symptoms.56 However, the evidence that weight control interventions are associated with improvements in asthma control remains controversial.57 58
Conditions that are part of the severe asthma syndrome
Asthma and rhinosinusitis often coexist and are believed to represent a spectrum of the same disease entity. In particular, in adults, chronic rhinosinusitis and nasal polyps have been shown to be important components of severe steroid-dependent asthma.59 Nasal symptoms and CT imaging of sinonasal involvement are related to asthma severity, sputum eosinophil counts and airflow limitation.60 Medical or surgical treatment of upper airway disease can improve asthma control. Patients with severe asthma should therefore be evaluated and treated for chronic rhinosinusitis, particularly if associated with nasal polyps.
Defining ‘problematic’, ‘difficult’ and ‘severe refractory’ asthma
By excluding factors that may aggravate or complicate asthma, the subgroup with truly severe refractory asthma can be defined and distinguished from patients with ‘problematic’ or ‘difficult’ asthma.
The term ‘problematic severe asthma’ includes all asthma and asthma-like symptoms that remain uncontrolled despite the prescription of high-intensity asthma treatment. It is an umbrella term that comprises patients with ‘difficult’ asthma as well as patients with ‘severe refractory’ asthma.
The term ‘difficult asthma’ is reserved for asthma that remains uncontrolled despite the prescription of high-intensity asthma treatment due to:
persistently poor compliance;
psychosocial factors, dysfunctional breathing, vocal cord dysfunction;
persistent environmental exposure to allergens or toxic substances;
untreated or undertreated comorbidities such as chronic rhinosinusitis, reflux disease or obstructive sleep apnoea syndrome.
The term ‘severe refractory asthma’ should be reserved for patients with asthma in whom alternative diagnoses have been excluded, comorbidities have been treated, trigger factors have been removed (if possible) and compliance with treatment has been checked, but still have poor asthma control or frequent (≥2) severe exacerbations per year despite the prescription of high-intensity treatment or can only maintain adequate control when taking systemic corticosteroids and are thereby at risk of serious adverse effects of treatment.
For this definition, poor asthma control is defined according to Juniper et al as a score of ≥1.5 by the 7-item Asthma Control Questionnaire61 62 or an equivalent score by any other standardised asthma control questionnaire. High-intensity treatment in adults is defined as ≥1000 μg/day fluticasone equivalent and/or daily oral corticosteroids combined with long-acting β2 agonists or any other controller medication. High-intensity treatment in school age children is defined as ≥500 μg/day fluticasone equivalent or daily oral corticosteroids combined with long-acting β2 agonists or any other controller medication. High-intensity treatment in pre-school children is defined as (1) high-dose ICS and oral leukotriene receptor antagonists at the time of viral exacerbations; and/or (2) ≥400 μg/day budesonide equivalent and oral leukotriene receptor antagonists given regularly.
Clinical and inflammatory phenotypes of severe asthma
Although the subgroup of patients with ‘severe refractory asthma’ is less heterogeneous than the group of patients with ‘difficult’ asthma, it is far from homogeneous and may be subdivided into different phenotypes.37 63 Phenotypes have been far less well studied in children, but it is likely that those described below are part of the spectrum of asthma, at least in older children.
From a clinical point of view, three categories of patients with severe asthma seem to be of particular importance: (1) those suffering from frequent severe exacerbations with relatively stable episodes between exacerbations (exacerbation prone asthma); (2) those who develop irreversible airflow obstruction (asthma with fixed airflow obstruction); and (3) those who depend on systemic corticosteroids for daily control of their asthma (steroid-dependent asthma).64
Exacerbation-prone asthma accounts for more than 40% of severe asthma in the SARP database,65 whereas 60% of patients in the TENOR study had evidence of fixed airflow limitation.66 These latter patients are less predisposed to severe exacerbations.67 Cohort studies in children suggest that some of these patients may have failed to increase their lung function adequately and fall off their lung function centiles,68 but many adult patients with severe asthma show accelerated decline of lung function,69 particularly men with recent non-allergic asthma.66 70
A subset of patients with severe asthma requires daily systemic corticosteroids to control their asthma at the cost of serious side effects. This might be due to relative insensitivity to corticosteroids or to involvement of the paranasal sinuses and distal airways in the inflammatory process.
From a pathological point of view, at least two phenotypes of severe asthma have been proposed, each associated with distinct clinical and pathophysiological characteristics. These subtypes include the persistent eosinophilic and non-eosinophilic forms of severe asthma.71
Severe asthma with persistent eosinophilia has been put forward by Wenzel and colleagues71 and further characterised by others.70–72 It is characterised by mixed eosinophilia and neutrophilia in bronchial biopsies and induced sputum despite the use of high-intensity ICS or oral corticosteroid treatment. This type of asthma is associated with severe exacerbations,8 9 sinus disease,72 involvement of the peripheral airways,71 airway remodelling73 and fixed airflow obstruction,70 and responds favourably to treatment with anti-interleukin 5 monoclonal antibody.9 10
In the non-eosinophilic subtype of severe asthma, airway eosinophils are either absent or suppressed by treatment in the presence of a high level of asthma symptoms.74 Airway inflammation in these patients with severe asthma is characterised by an increased percentage of neutrophils.75 76 The potential causal factors that induce airway neutrophilia are numerous, but it is still uncertain whether these cells play an active role in an ongoing airway damaging process.
Different phenotypes of severe asthma have also been proposed in children.77 In general, children with severe asthma have no gender bias and are highly atopic with relatively well-preserved lung function. Subphenotyping has been mainly by inflammatory cells in induced sputum. Airway eosinophilia might be characteristic for a separate exacerbating phenotype in which food allergy is a potential factor increasing the severity of exacerbations.78
Towards new phenotypes of severe refractory asthma
Clinical characterisation of patients by a single clinical characteristic or biomarker is probably not enough to describe the severe asthma phenotypes. The fact that, at a group level, clinical and pathophysiological biomarkers do not correlate strongly with one another79 suggests that they add independent information about a patient's underlying phenotype. New approaches to statistical modelling, such as cluster analysis, may enable a better definition of asthma phenotypes. The first study using factor analysis in asthma supported the idea that different dimensions of the disease—such as airway obstruction, hyper-responsiveness and eosinophilic inflammation—independently contribute to the disease.80 Two other more recent studies identified different clusters of refractory asthma.67 81 The first study distinguished three clusters, one characterised by concordance between asthma symptoms and eosinophilic airway inflammation (early-onset atopic asthma) and two clusters with marked discordance between symptom expression and eosinophilic airway inflammation (obese women with symptom-predominant asthma and late-onset inflammation-predominant asthma).81 The other study identified three clusters of patients with severe refractory asthma: one cluster of obese women with late-onset non-atopic asthma, moderate reductions in FEV1 and frequent oral corticosteroid use to manage exacerbations, and two other clusters with severe airflow obstruction and bronchodilator responsiveness who differed in their ability to attain normal lung function, age of asthma onset, atopic status and use of oral corticosteroids.67
U-BIOPRED
The pan-European project Unbiased Biomarkers for the Prediction of Respiratory Disease Outcome (U-BIOPRED),82 as part of the Innovative Medicines Initiative,83 will push this further by integrating high dimensional data from invasive (bronchial biopsies), non-invasive (blood, sputum, exhaled air) and patient-reported outcomes into distinct phenotype handprints by using an innovative systems biology approach.84 This will enable more detailed phenotyping of adult and paediatric severe asthma and prediction of therapeutic efficacy in view of tailored management.
Conclusion
Over the last 15 years there has been a lack of consensus about the definition and diagnosis of severe asthma. Research studies in patients with severe asthma have used different inclusion and exclusion criteria and the nomenclature to describe these patients has been quite confusing. There is now increasing evidence that patients with ‘severe asthma’ form a heterogeneous group, and that many aggravating factors may influence the clinical presentation. For the development of innovative therapies, there is an urgent need for an accurate characterisation of patients with truly severe refractory asthma and for subphenotyping these patients. The U-BIOPRED programme not only reached international consensus on the definition and diagnosis of severe asthma but, more importantly, produced for the first time a stepwise algorithm by which the patient with truly severe refractory asthma may be identified.
References
Footnotes
List of contributors at the U-BIOPRED International Consensus Definition and Diagnosis of Severe Asthma: Bel EH, Bergeron C, Bisgaard H, Bleecker E, Boulet L-P, Bousquet J, Brightling CE, Bush A, Castro M, Chanez P, Chung KF, Compton CH, Cookson W, de Boer WI, Djukanovic R, Fleming L, Gaga M, Hedlin G, Howarth PH, Ivanoff N, Kiley J, Larsson LG, Menzies-Gow A, Meyers DA, Myles D, Nething K, O'Byrne PM, Palkonen S, Polosa R, Purkins L, Rohou S, Serdrevic D, Sousa A, Sterk PJ, Ventresca G, Versnel J, Wagener AK, Wagers SS, Wenzel SE.
Funding This report was supported by grants from the European Commission Innovative Medicine Initiative Understanding Severe Asthma, IMI_Call_2008_1_12; final protocol available at http://www.imi.europa.eu/.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.