Article Text
Abstract
Rationale Airway inflammation in asthma is heterogeneous with different phenotypes. The inflammatory cell phenotype is modified by corticosteroids and smoking. Steroid therapy is beneficial in eosinophilic asthma (EA), but evidence is conflicting regarding non-eosinophilic asthma (NEA).
Objectives To assess the inflammatory cell phenotypes in asthma after eliminating potentially confounding effects; to compare steroid response in EA versus NEA; and to investigate changes in sputum cells with inhaled corticosteroid (ICS).
Methods Subjects undertook ICS withdrawal until loss of control or 28 days. Those with airway hyper-responsiveness (AHR) took inhaled fluticasone 1000 μg daily for 28+ days. Cut-off points were ≥/<2% for sputum eosinophils and ≥/<61% for neutrophils.
Results After steroid withdrawal (n=94), 67% of subjects were eosinophilic, 31% paucigranulocytic and 2% mixed; there were no neutrophilic subjects. With ICS (n=88), 39% were eosinophilic, 46% paucigranulocytic, 3% mixed and 5% neutrophilic. Sputum neutrophils increased from 19.3% to 27.7% (p=0.024). The treatment response was greater in EA for symptoms (p<0.001), quality of life (p=0.012), AHR (p=0.036) and exhaled nitric oxide (p=0.007). Lesser but significant changes occurred in NEA (ie, paucigranulocytic asthma). Exhaled nitric oxide was the best predictor of steroid response in NEA for AHR (area under the curve 0.810), with an optimum cut-off point of 33 ppb.
Conclusions After eliminating the effects of ICS and smoking, a neutrophilic phenotype could be identified in patients with moderate stable asthma. ICS use led to phenotype misclassification. Steroid responsiveness was greater in EA, but the absence of eosinophilia did not indicate the absence of a steroid response. In NEA this was best predicted by baseline exhaled nitric oxide.
- Asthma
- inflammatory cell
- eosinophil
- neutrophil
- phenotype
Statistics from Altmetric.com
Introduction
Asthma phenotypes may be described both clinically and pathologically and are heterogeneous. Increasing emphasis is being given to categorising patients in relation to inflammatory phenotype using induced sputum analysis. This may provide insights into both the natural history1 and the potential for treatment response.2 Four subtypes have been identified—eosinophilic, neutrophilic, paucigranulocytic or mixed cellularity—depending on the presence or absence of sputum eosinophils and/or neutrophils.3 A simpler classification provides for two categories—eosinophilic asthma (EA) and non-eosinophilic asthma (NEA).
EA is associated with atopy and IgE-mediated eosinophilic inflammation. In general, corticosteroid therapy provides for benefits in patients with EA.4 5 It is considered to be the commonest pathological subtype, although this perception is not strictly justified. In one review, the prevalence of EA ranged from 88% to as low as 22% of cases.6
NEA has been demonstrated in persistent asthma of all grades of severity.1 3 7–9 In these studies neutrophil predominance has been identified. Neutrophilic inflammation has also been demonstrated in severe asthma10 and in acute exacerbations.11 In contrast to EA, there is conflicting evidence regarding the efficacy of inhaled corticosteroids (ICS) in NEA. Some studies have shown NEA to be relatively steroid-unresponsive.5 9 12 13 Conversely, other studies have shown similar degrees of steroid responsiveness in both EA and NEA.14 15
Several important background issues may influence the accuracy of induced sputum cell counts and hence phenotype classification. First, there is the possibility that the inflammatory cell phenotype varies longitudinally over time. We will not address this issue further in this paper. Second, there are the potential effects of factors such as steroid use and cigarette smoking. Concurrent use of ICS therapy is potentially critical. Ideally, patients should be steroid-free but, because withdrawing ICS carries risk, it has been avoided in the majority of studies. The effects of steroids may also be relevant in neutrophilic asthma. Corticosteroids are known to prolong the survival of functional neutrophils, at least in vitro,16 and thus the results of studies in which neutrophil predominance is reported may have been confounded.1 3 7 8 10 11 Smoking may also lead to increased sputum neutrophilia.17 Other potential confounders include age,18 air pollution,19 occupation,20 high-grade exercise,21 recent viral respiratory infection,22 bronchiectasis8 and gastro-oesophageal reflux.23
The aims of this study were (1) to assess the prevalence of eosinophilic, neutrophilic, mixed and paucigranulocytic patients with asthma after eliminating modifying factors; (2) to compare the therapeutic responses to ICS in EA versus NEA phenotypes; and (3) to investigate any changes in inflammatory phenotype classification which might occur with ICS therapy. The study was carried out in patients in whom ICS treatment was withdrawn and later recommenced, and in whom cigarette smoking was excluded. It was conducted in the context of obtaining run-in data for larger ongoing clinical trials in which establishing inflammatory phenotype and steroid responsiveness were prerequisites (Australian New Zealand Clinical Trials Registry ACTRN12606000531516, ACTRN12606000488505).
Methods
A more detailed description of the methods used is found in the online supplement.
Patients
Patients aged 18–75 years with stable persistent asthma were enrolled. Exclusion criteria included: respiratory infection in preceding 4 weeks; >10 pack-year smoking history or smoking in the previous 3 months; use of oral prednisolone in previous 3 months; other pulmonary disease or significant comorbidity.
Study design
The study comprised two phases: run-in and steroid withdrawal (phase 1) and a trial of steroid (phase 2) as shown in table 1.
Study plan
Baseline measurements and withdrawal of ICS (phase 1)
At the initial visit, all participants gave written informed consent. Demographic and medical data were obtained. Measurements included peak expiratory flow, fraction of exhaled nitric oxide (Feno), spirometry and bronchodilator response, skin prick testing and total IgE. Subjects received a symptom diary, peak flow meter, an emergency prednisolone supply, albuterol inhaler, large volume spacer and a contact details card. Diaries were completed for 2 weeks while subjects continued on their usual medications and they recorded morning and evening peak flow, bronchodilator use, night wakening with asthma symptoms and asthma symptom score.
Following the run-in period, individualised criteria for ‘loss of control’ (LOC) were generated (see table E1 in online supplement). Subjects completed validated questionnaires to assess asthma control (Asthma Control Questionnaire (ACQ) and Asthma Control Test (ACT)) and quality of life (Asthma Quality of Life Questionnaire with standardised activities (AQLQ)). ICS and long-acting β agonists were withdrawn and subjects were reviewed regularly by telephone contact until either LOC or 28 days, whichever came sooner, at which time the next visit was scheduled. LOC was deemed to have occurred when one or more of the pre-set criteria were met. At LOC, testing took place over 2–4 consecutive days: first, to define the inflammatory phenotype in a population which was steroid-free; and second, to make baseline measurements against which the effectiveness of the trial of steroid could be measured. Where LOC was deemed to be due to respiratory infection, the patient was excluded. Testing included hypertonic saline challenge ± methacholine challenge ± spirometry with bronchodilator response. Subjects proceeded to phase 2 if they had a provocative dose of hypertonic saline causing a 15% fall in forced expiratory volume in 1 s (FEV1) of <12 ml (PD15 <12 ml hypertonic saline), a provocative dose of methacholine causing a 20% fall in FEV1 of <8 μmol (PD20 <8 μmol methacholine) or ≥12% improvement in FEV1 post-bronchodilator.
Trial of steroid (phase 2)
Patients were given fluticasone (Flixotide, GlaxoSmithKline, Greenford, UK) 1000 μg daily via a spacer for 28+ days during which they completed the daily diary. ACQ, ACT, AQLQ, Feno, spirometry, sputum induction and adenosine monophosphate (AMP) challenge were carried out in sequential order before and after treatment. Steroid responsiveness was defined as one or more of the following: ≥12% increase in FEV1; ≥0.5 point decrease in ACQ; ≥2 doubling dose increase in PC20AMP; ≥40% decrease in Feno.
Study procedures
After measurement of airway hyper-responsiveness (AHR) to hypertonic saline, induced sputum was immediately collected, the whole sample was processed and a non-squamous cell differential obtained. All cell counts were read and agreed by two trained observers. A cut-off point of ≥2% eosinophils was used to define EA and <2% to define NEA.24 Eosinophilic, neutrophilic, mixed and paucigranulocytic inflammation were defined using cut-off points of ≥/<2% for sputum eosinophils24 and ≥/<61% for sputum neutrophils.3 A panel of cytokines as well as neutrophil elastase (NE) were measured in sputum supernatant. Standardised protocols were used for methacholine and AMP challenges.
Statistical analysis
Comparisons between subjects with EA and those with non-EA were made before and after steroid withdrawal using unpaired t tests and Mann–Whitney U tests for continuous data and χ2 tests for categorical data. Feno, PD15 hypertonic saline, PC20AMP, % percentages of eosinophils, bronchoepithelial cells and lymphocytes and total cell counts were analysed after logarithmic transformation. A comparison of steroid responsiveness between patients with EA and those with NEA was made by mixed model analysis of continuous variables. χ2 tests were used to compare the proportions of patients with EA and NEA with clinically significant improvements in ACQ, FEV1 and PC20AMP after treatment using the predefined cut-off points. Receiver operating characteristic curves were used to compare predictors of steroid responsiveness and sensitivities, specificities, positive and negative predictive values and accuracy were calculated.
Results
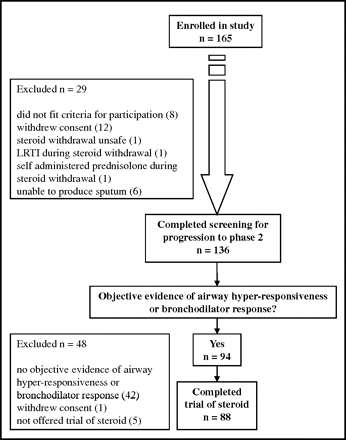
Of a total of 165 individuals screened, 94 had objective evidence of AHR or reversible airflow obstruction; 88 individuals completed the trial of inhaled fluticasone (figure 1). The baseline characteristics of the 94 participants are shown in table 2. Seventy-four were taking regular ICS. Of these, 52 (70%) lost control after steroid withdrawal.
Consort diagram outlining the selection and treatment allocation of patients. LRTI, lower respiratory tract infection.
Baseline characteristics of all subjects subsequently categorised as eosinophilic asthma (EA) and non-eosinophilic asthma (NEA)
After steroid withdrawal, 65 subjects (69%) were classified as EA and 29 (31%) as NEA. Those classified as EA were more often taking ICS (EA: 86%, NEA: 62%, p=0.008) and at higher doses (EA: 856±620 μg; NEA: 397±478 μg, p=0.001) at baseline. Of the 56 with EA taking regular ICS, 46 (82%) experienced LOC after steroid withdrawal compared with 6 of 18 (33%) with NEA (p<0.001; see figure E1 in online supplement).
Changes in symptoms and lung function with steroid treatment
Table 3 shows the results for ACQ, ACT, AQLQ, peak expiratory flow, FEV1, PC20AMP and Feno after steroid withdrawal (see table E2 in online supplement) and subsequently after treatment for 28+ days with inhaled fluticasone. The effect of fluticasone was significant for all measured parameters except mean morning peak flows. The response to treatment was significantly greater in patients with EA than in those with NEA for all parameters except mean morning peak flows and FEV1.
Changes in symptoms (ACQ, ACT, AQLQ), lung function (morning PEF, FEV1), airway hyper-responsiveness (PC20AMP) and airway inflammation (Feno) in eosinophilic asthma (EA) and non-eosinophilic asthma (NEA) after inhaled fluticasone (1000 μg daily) given for 28+ days
Using predetermined cut-off points for clinically significant change, steroid responsiveness was significantly more frequent in EA than in NEA for ACQ (p=0.001), FEV1 (p<0.001), PC20AMP (p=0.008) and Feno (p<0.001) (table 4). Analyses using cut-off points of 1% and 3% to define EA provided similar results, although the differences between EA and NEA were of lesser magnitude (see tables E8 and E9 in online supplement).
Categorical analysis of steroid response in eosinophilic asthma (EA) and non-eosinophilic asthma (NEA) using predetermined cut-off points for improvements in symptoms, lung function, airway hyper-responsiveness and airway inflammation
Changes in inflammatory cell phenotypes with steroid treatment
After steroid withdrawal, 63 subjects (67%) were eosinophilic, 29 (31%) were paucigranulocytic and 2 (2%) were mixed. There were no neutrophilic subjects (table 5). Similar results were obtained using cut-off points of ≥/<1% or ≥/<3% for sputum eosinophils. After fluticasone, 32 (51%) of the original eosinophilic subjects remained eosinophilic while 22 (35%) became paucigranulocytic, 2 (3%) became mixed and 2 (3%) became neutrophilic. Twenty-one (72%) of the original paucigranulocytic subjects remained paucigranulocytic while 4 (14%) became eosinophilic and 3 (10%) became neutrophilic. Thus, a total of 5 subjects (5%) were designated neutrophilic following steroid. One of the subjects originally with the mixed subtype did not change while the other became eosinophilic.
Sputum phenotype at loss of control (LOC) or 28 days after steroid withdrawal and after fluticasone 1000 μg daily for 28+ days
After steroid withdrawal there was no difference in the neutrophil proportions between patients with EA and those with NEA (see table E2 in online supplement). As expected, eosinophils decreased significantly in EA with fluticasone from 17.9% (95% CI 14.1% to 22.8%) to 3.6% (95% CI 2.3% to 5.8%), but did not change in NEA (p<0.001). However, for all subjects there was an increase in sputum neutrophils with fluticasone from 19.3% (95% CI 15.7% to 22.9%) to 27.7% (95% CI 23.1% to 32.4%) (p=0.024), but this was not significantly different between EA and NEA (table 6). There was a significant relationship between age and percentage of sputum neutrophils (r=0.33, p<0.001), which persisted with fluticasone (r=0.26, p=0.014). There were no significant correlations between sputum cell counts and body mass index.
Changes in sputum cells in eosinophilic asthma (EA) and non-eosinophilic asthma (NEA) after inhaled fluticasone (1000 μg daily) for 28+ days
At LOC or 28 days after steroid withdrawal, EA was characterised by higher levels of interleukin (IL)-1β (p=0.033), IL-5 (p<0.001), IL-6 (p<0.001), IL-8 (p=0.014) and IL-10 (p=0.014) in sputum supernatant compared with NEA (see table E3 in online supplement). With fluticasone, IL-8 increased significantly from a median of 622.9) pg/ml (IQR 352.2–698.4) to 2207.2 pg/ml (IQR 936.8–4925.9) (p<0.001) and and NE increased significantly from a median of 101.8 ng/ml (IQR 78.2–134.0) to 160.0 ng/ml (IQR 123.7–208.5) (p<0.001).
Predictors of steroid responsiveness in NEA
In order to explore whether objective measurements at baseline or LOC might predict steroid responsiveness in the NEA group, receiver operating characteristic analyses were carried out. Areas under the curve are shown in table 7 and relevant comparisons are shown in tables E4, E5 and E6 in the online supplement. FEV1 and PC20AMP were predictors of an increase in FEV1. Feno was the best predictor of steroid response as defined by an improvement in PC20AMP (see table E4 in the online supplement), with an optimum cut-off point of 33 ppb (see table E7 in the online supplement). None of the measured parameters was able to predict an improvement in ACQ.
Areas under the curve (AUC) for receiver operator characteristic analyses in which measurements of FEV1 at baseline, FEV1 at loss of control (LOC) or 28 days after steroid withdrawal, change in FEV1 with bronchodilator at baseline, AHR as measured by PD15HS and by PC20AMP and Feno were used as predictors
Discussion
In this study we have clarified important issues pertaining to the classification of inflammatory phenotypes in asthma and their relationship to steroid response. Our principal findings are: (1) after steroid withdrawal we were unable to identify a neutrophilic phenotype in our population of patients; (2) ICS treatment was associated with increased airway neutrophils and a switch to a neutrophilic phenotype in some patients; (3) although steroid responsiveness is greater in EA, it is not exclusive to this phenotype but also occurs in NEA; and (4) Feno may be used to predict the steroid response in NEA.
Our finding of the absence of a neutrophilic phenotype after steroid withdrawal and, with steroid, of a significant increase in the proportion of sputum neutrophils (∼10%) and in the number of patients with neutrophilic asthma has significant implications. It may be that in previous studies reporting inflammatory phenotypes the results are not strictly accurate because patients were receiving corticosteroid.1 3 7 8 10 11 While discontinuing treatment may be impractical, the modifying effect of steroid should be taken into account. The same is true for studies including smokers9 in whom neutrophilia is more marked. In one study the sputum neutrophil count was 23% in non-smokers and 47% in smokers.17 The neutrophilic phenotype is more widely reported in severe asthma.10 This may in part reflect the extent to which patients have greater steroid exposure. Other factors such as exercise21 and respiratory infection22 may influence sputum cells; these were excluded from our study. The demonstration of neutrophilic inflammation1 3 7–10 has prompted the suggestion that the neutrophil may be a key effector cell in NEA25 and thus a target for novel therapies. Such speculation may be less appropriate.
Our findings are consistent with evidence that steroid prolongs survival of functional neutrophils.16 In human studies, neutrophilia is greater in steroid-dependent intractable asthma than in non-steroid-dependent intractable asthma.26 Neutrophils in endobronchial biopsies increased with steroid (from 43.5 to 150.8 cells/mm2, p<0.001)27 and after prednisolone (from 76 to 140 cells/mm2, p=0.05).28 The greater increase in neutrophils seen in these studies may reflect the fact that different tissues were sampled. It is not clear whether neutrophils associated with steroid exposure are activated or are innocent bystanders. We have shown that IL-8, a neutrophil chemoattractant, is increased in sputum supernatant after steroid, consistent with the finding that IL-8 mRNA expression is increased after oral methylprednisolone.29 Our finding of a significant increase in NE with treatment suggests that resident neutrophils retain functional capacity.
Our study showed that, although the steroid response was significantly greater in EA, it was not unique to that phenotype. Although the presence of eosinophilia indicates greater likelihood of steroid response in airways disease,4 our results suggest that the absence of eosinophilia does not imply the absence of treatment response. A proportion of subjects with NEA showed significant improvements with fluticasone: reduced symptoms, 46%; increased airway calibre, 14%; reduced AHR, 43% (table 4). This picture is consistent with data from Godon et al14: 15/46 patients with sputum eosinophils <1% had an improvement in AHR with fluticasone. Similarly, in another study the benefits of systemic steroid treatment were independent of the pretreatment sputum eosinophil count.15 In contrast, in a randomised trial using mometasone, Berry et al reported no significant steroid-related improvements in symptoms or AHR in non-eosinophilic subjects.13 Overall, the inconsistencies in these data suggest that it is probably unwise to categorise NEA as a distinct steroid-unresponsive entity. Perhaps the distinction between EA and NEA based on a specific cut-off point for sputum eosinophils is a false one. NEA (ie, paucigranulocytic rather than neutrophilic) may be a milder form of the same pathology but without eosinophil trafficking into the airway lumen. The presence of eosinophils is therefore informative, but their absence is not necessarily a reliable indicator regarding steroid response.
Predicting steroid responsiveness in airways disease is important. Not surprisingly, in our study FEV1 was the best predictor of increased airflow with treatment in both EA and NEA (table 7). No pretreatment test was helpful in predicting improved symptoms in NEA. However, Feno was the most useful predictor of steroid response in NEA as measured by a reduction in AHR, with a cut-off point of 33 ppb giving the best predictive accuracy. As far as we are aware, this finding is novel and is surprising given that Feno is regarded as a surrogate marker for airway eosinophilia.30 The high predictive value of Feno for improved AHR with fluticasone in NEA reinforces earlier findings regarding the predictive value of Feno measurements in steroid-naïve subjects.31
A limitation of our study is that a placebo-controlled design was not used for the steroid trial. This was for ethical reasons. Seventy per cent of patients taking ICS lost control after treatment withdrawal. It would have been inappropriate to treat these patients with placebo for up to 28 days beyond the point of LOC. An alternative would have been to select only patients able to tolerate ICS withdrawal. However, this would have resulted in selection bias; only patients with mild asthma would have been eligible and the data obtained would have been less generalisable. It is possible that, because the trial was not placebo-controlled, the significance of the changes in symptoms is questionable. Similarly, although we cannot discount that individual inflammatory cell profiles might regress to the mean or change with time, in the absence of exacerbations or changes in treatment this seems unlikely.32
Our method for sputum analysis was to use the whole sample rather than selected sputum plugs. Although caution is required when making comparisons between this and other studies in which the alternative method is used, whole sample processing is well validated and provides comparable results.24 Pizzichini et al33 confirmed that there are no significant differences in the cell proportions when comparing selected versus residual sputum. A priori we chose a cut-off point of ≥2% eosinophils to define EA.24 This was based on the work of Belda et al34 and Spanavello et al35 who showed that, in normal subjects, the mean plus 2SD for sputum eosinophils is approximately 2%. We re-analysed our data using cut-off points of 1% and 3% but this had no major effect on the overall results. In fact, a cut-off point of 2.3% was best for predicting changes in FEV1 (see table E10 in the online supplement).
In conclusion, steroid therapy contributes to increased airway neutrophilia as well as reduced eosinophilia. The inflammatory cell phenotypes reported in previous studies may therefore be inaccurate, influenced by the effects of steroid exposure and smoking. While identifying the eosinophilic phenotype is important, it is not definitive for determining the response to steroid therapy. Modified responses to corticosteroid may still occur in patients with NEA (ie, paucigranulocytic asthma) and can be predicted using Feno measurements. Further comments are included in the online supplement.
Acknowledgments
We thank Miss Sarah Featherston for her administrative support, Dr Sarah Young for her expertise in measurement of sputum supernatant fluid mediators and Associate Professor G Peter Herbison and Dr Erik Landhuis for their statistical advice.
References
Supplementary materials
Web only Data thx.2009.126722
Files in this Data Supplement:
Footnotes
Linked articles 131391.
Funding Lottery Health New Zealand.
Competing interests None.
Ethics approval This study was conducted with the approval of the Lower South Regional Ethics Committee and all patients gave written informed consent.
Provenance and peer review Not commissioned; externally peer reviewed.