Article Text
Abstract
Background This randomised, double-blind, placebo controlled, four-period crossover study assessed the efficacy and safety of once-daily QVA149, a dual bronchodilator consisting of the long-acting β2-agonist indacaterol and the long-acting muscarinic antagonist glycopyrronium (NVA237), in patients with moderate to severe chronic obstructive pulmonary disease (COPD).
Methods Patients (N=154) were randomly assigned to receive QVA149 (indacaterol/NVA237) 300/50 μg, indacaterol 300 μg, indacaterol 600 μg, or placebo, once daily for 7 days with a 7-day washout period between each treatment. The primary endpoint was trough forced expiratory volume in 1 s (FEV1) (mean of 23 h 15 min and 23 h 45 min post-dose values) on day 7. Other endpoints included trough FEV1 on day 1, individual time point FEV1 and monitoring and recording of all adverse events.
Results A total of 135 (87.7%) patients completed the study (all randomly assigned patients: mean age 61.7 years, 61.4% male, post-bronchodilator FEV1 52.2% predicted, FEV1/forced vital capacity 47.6%). The estimated treatment difference (95% CI) for trough FEV1 on day 7 between QVA149 and placebo was 226 ml (192 to 260; p<0.001). The estimated treatment difference between QVA149 and indacaterol 300 and 600 μg was 123 ml (89 to 157; p<0.001) and 117 ml (83 to 150; p<0.001), respectively. The improvements in mean trough FEV1 exceeded the predefined minimal clinically important differences of 100–140 ml for QVA149 versus placebo and indacaterol. Similar results were observed on day 1. All treatments were well tolerated.
Conclusions QVA149 demonstrated rapid and sustained bronchodilation with significant improvements compared with indacaterol monotherapy and placebo in patients with COPD.
Clinical trial registration NCT00570778.
- bronchodilation
- combination therapy
- COPD pharmacology
- glycopyrronium
- indacaterol
- NVA 237
- QVA149
Statistics from Altmetric.com
Bronchodilators are the mainstay for the treatment of chronic obstructive pulmonary disease (COPD).1 The long-acting β2-agonists (LABA), formoterol and salmeterol, and the long-acting muscarinic antagonist (LAMA) tiotropium are widely used as maintenance treatment for COPD. When symptoms are not adequately controlled by monotherapy, combining bronchodilators of different classes, in particular an inhaled muscarinic antagonist with a β2-agonist, is associated with better outcomes.1
Combining LABA with anticholinergic agents has been shown to be pharmacologically useful because β2-agonists decrease the release of acetylcholine, leading to consequent amplification of the bronchial smooth muscle relaxation induced by the anticholinergic agent.2 The addition of an anticholinergic agent can also reduce peripheral bronchoconstrictor effects of acetylcholine, consequently causing amplification of bronchodilation elicited by the β2-agonist through direct stimulation of smooth muscle β2-adrenoceptors.2 The superior bronchodilation obtained by combining bronchodilators with different mechanisms of action may be attributed to complementary pharmacodynamic profiles whereby the anticholinergic causes prolonged bronchodilation and the LABA contributes to bronchodilation and a rapid onset with greater peak effect.3–5 A number of studies have shown LABA/LAMA combinations to improve bronchodilation significantly compared with either agent used alone.6–10
Most studies published to date have reported on the combination of a twice-daily LABA with tiotropium3 6–14 or twice-daily LABA with short-acting muscarinic antagonists.15 16 Although formoterol is not approved for once-daily administration, a combination of tiotropium and formoterol given once daily provided additional benefit over either formoterol twice daily and tiotropium once daily.6 In addition, the most favourable bronchodilation was achieved with this combination and significantly higher peak and average forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) responses were observed compared with either component. Therefore, a combination of a once-daily LABA and a once-daily LAMA should provide further benefits over 24 h and may have the potential to improve patient convenience and compliance and therefore improve outcomes.
QVA149, an inhaled combination of two 24-h bronchodilators, the LABA indacaterol and the LAMA NVA237, is in development for COPD. The efficacy of both component monotherapies as once-daily therapies has been demonstrated, and both have been shown to be safe and well tolerated.17–23 The aim of the current study was to examine the bronchodilatory effect and safety of QVA149 in patients with moderate to severe stable COPD.
Methods
Subjects
Male or female patients (≥40 years) with moderate to severe COPD1 and smoking history of 10 pack-years or greater were enrolled. Patients with a post-bronchodilator FEV1 of 30% or greater and less than 80% of predicted normal and post-bronchodilator FEV1/FVC less than 0.70 (both measurements taken 30 min following inhalation of 4×100 μg puffs of salbutamol) were included. Exclusion criteria were the following: requiring daily oxygen therapy, hospitalisation for an exacerbation of airway disease in 6 weeks before study start, respiratory tract infection, any history of asthma (including childhood asthma), prolonged QTc interval, or any other clinically relevant medical conditions. Pregnant or nursing (lactating) women, and women of child-bearing potential, unless using acceptable methods of contraception, were excluded. Patients with a history of untoward reactions to any of the study drugs or unable to use a single-dose dry powder inhaler device or a pressurised metered dose inhaler (rescue medication) or perform spirometry measurements were excluded.
Treatment with any bronchodilators other than those prescribed in the study and inhaled corticosteroids (ICS) were not permitted during the study. During screening, LABA were changed to regular short-acting β2-agonists. Salbutamol was permitted only as rescue medication during the treatment period. The steroid component of any fixed-dose combination therapy was to be replaced with an equivalent dose of ICS administered as a single agent. Patients previously treated with an ICS as a single agent (ie, not in a fixed-dose combination) were to continue on their pre-study inhaled steroid regime.
Study design
This was a randomised, double-blind, placebo controlled, four-period crossover, multicentre study. Following screening, eligible patients were randomly assigned to a sequence of treatments with the following once-daily regimens: QVA149 (indacaterol/NVA237) (300/50 μg); indacaterol 300 μg; indacaterol 600 μg or placebo, all administered by means of a single-dose dry powder inhaler (Breezhaler®*). The study had four 7-day treatment periods with a 7-day washout period between each treatment. The study protocol was reviewed and approved by the independent ethics committee or institutional review board of each participating centre. The study was conducted according to the Declaration of Helsinki. Written informed consent was obtained from each subject before enrolment.
Assessments
The primary endpoint was trough FEV1 on day 7, defined as the mean of the 23 h 15 min and 23 h 45 min post-dose FEV1 values. Other variables included trough FEV1 on day 1, trough FVC at days 1 and 7, individual time point FEV1 and FVC on days 1 and 7. Pre-dose spirometry measurements on the various study days were taken in the morning between 08:00 and 10:00 hours, and all subsequent assessments were scheduled for the same clock time. Spirometry measurements were taken in a central laboratory using a Vitalograph 6800 machine provided by Biomedical Systems, Brussels, Belgium. Up to five efforts per time point were measured and experiments were to be repeatable at least three times per the American Thoracic Society/European Respiratory Society guidelines. In addition, changes in trough and individual time point inspiratory capacity (IC) were measured. FEV1 and FVC were measured at 45 and 15 min before the first dose of study treatment (baseline), and at 5, 15 and 30 min, 1, 2, 3, 4 h, 23 h 15 min and 23 h 45 min post-dose on day 1. On day 7, FEV1 and FVC were performed at 45 and 15 min pre-dose and 5, 15, 30 min, 1, 2, 3, 4, 5, 6, 8, 10, 12, 23 h 15 min and 23 h 45 min post-dose. Standardised FEV1 area under the curve (AUC) between 5 min and 4 h post-dose (AUC5min–4h), and 5 min and 23 h 45 min post-dose (AUC5min–23h 45min) on day 1 and between 5 min and 4 h post-dose (AUC5min–4h), 5 min and 12 h post-dose (AUC5min–12h) and 5 min and 23 h 45 min post-dose (AUC5min–23h 45min) on day 7 were also determined. Peak FEV1 (defined as the maximum FEV1 value from 5 min to 4 h post-dose) was measured on days 1 and 7 of each treatment period. IC measurements were taken for the scheduled post-dose time points: 30 min, 1, 2 and 4 h on day 1 and day 7, at 8 and 12 h on day 7 of each treatment period. Safety assessments consisted of recording all adverse events (AE) and serious AE, with their severity, duration and relationship to the study drug. In addition, regular monitoring of haematology, blood chemistry and urine, vital signs (pulse rate, blood pressure), physical condition and body weight were assessed. ECG measurements were also made before and after taking the study drug.
Statistical analysis
Patients were randomly assigned to one of the treatment sequences using an automated system. Blinding was to be maintained from randomisation until database lock unless any patient emergencies arose. The efficacy analysis population used a treatment-as-received convention, whereas the safety population used a treatment-as-assigned convention. The modified intent-to-treat (mITT) population included all randomly assigned patients who received at least one dose of the study drug. The mITT and safety populations were the same except that the safety population allowed the inclusion of non-randomly assigned patients who received the study drug by error. The primary analysis population for efficacy was the mITT population.
The primary variable, trough FEV1 on day 7, was analysed using an analysis of covariance with the following model.
Trough FEV1 at day 7=sequence effect+patient (sequence)+period effect+treatment effect+(period) baseline FEV1+error. A similar model was used for other efficacy variables.
A difference of 120 ml in trough FEV1 between QVA149 300/50 μg and placebo was considered a clinically important difference for COPD patients.24 A sample size of 36 evaluable patients was required to detect this difference between QVA149 300/50 μg and placebo as statistically significant at the 5% significance level (two-sided) with 90% power. A difference of 60 ml in trough FEV1 between QVA149 300/50 μg and indacaterol 300 μg was considered a clinically important difference for COPD patients.
A sample size of 112 evaluable patients was required to detect this difference between QVA149 300/50 μg and indacaterol 300 μg as statistically significant at the 5% significance level (two-sided) with 85% power. Assuming a drop-out rate of 20%, a sample size of 140 patients was chosen to provide greater than 99% power for the primary endpoint (trough FEV1, QVA149 300/50 μg vs placebo) and 85% power for the key secondary endpoint (trough FEV1, QVA149 300/50 μg vs indacaterol 300 μg). Least squares mean, standard errors and associated 95% CI for QVA149 300/50 μg and placebo are presented. The estimated treatment differences for QVA149 versus placebo were presented along with the associated 95% CI and p value (two-sided). Adjustment for multiplicity was made through a hierarchial test approach for the analyses of primary and key secondary variables. First, the superiority of QVA149 over placebo was evaluated using trough FEV1 on day 7. If QVA149 was found to be superior over placebo, the superiority of QVA149 over indacaterol was evaluated in a similar manner. Assessments of vital signs and QTc (Fridericia's) were analysed using analysis of covariance.
Results
Patient disposition
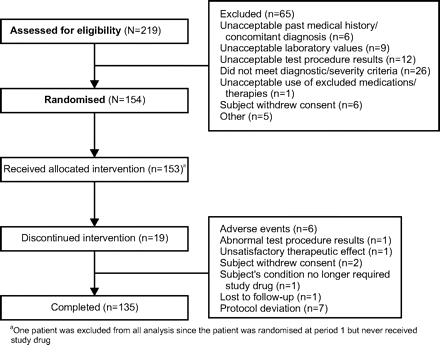
A total of 154 patients was randomly assigned and 135 (87.7%) completed the study (figure 1). A total of 19 patients discontinued the study, with the majority (n=10) of the discontinuations occurring during the first treatment period. The main reasons for premature withdrawal were protocol deviations, AEs, and either received study drug and not randomised or randomised but did not receive study drug.
Patient disposition.
Patient demographics and baseline clinical characteristics
Patient demographics and baseline characteristics are summarised in table 1. The mean age of all patients was 61.7 years; the majority of patients were male (61.4%) and Caucasian (98.7%). The duration of COPD was on average 7.9 years (table 1).
Patient demographics and baseline characteristics
The average pre-bronchodilator FEV1 in the study was 1.3 l (FEV1 percentage predicted: 45.3) and the average FEV1 reversibility was 17.2%. Patients showed a relatively high reversibility at baseline (17.2%) despite any history of asthma or childhood asthma being an exclusion criterion. The post-bronchodilator FEV1/FVC ratio was 47.6% and the mean post-bronchodilator FEV1 percentage predicted was 52.2%, indicating that the patient population comprised moderate to severe COPD patients with airflow limitation that was not fully reversible. Approximately half of the patients were ex-smokers with the remainder being current smokers. Baseline ECG were reported as normal or as having clinically insignificant abnormalities.
Efficacy
The least squares mean trough FEV1 on day 7 for QVA149 300/50 μg was superior to placebo (difference 226 ml) and indacaterol 300 and 600 μg (difference 123 and 117 ml, respectively) (p<0.0001). Likewise, QVA149 was superior (p<0.0001) to all other treatments on day 1 (figure 2A, B).
Trough forced expiratory volume in 1 s (FEV1) on (A) day 7 (B) day 1.
QVA149 showed a fast onset of action (5 min post-dose on day 1) with a significant (p<0.0001) increase in FEV1 over placebo, indacaterol 300 μg and indacaterol 600 μg. A treatment difference of 141 ml was observed between QVA149 and placebo at 5 min post-dose on day 1. QVA149 also showed a significant (p<0.05) increase in FEV1 over placebo, indacaterol 300 μg and indacaterol 600 μg at all other time points on day 1. Statistically significant (p<0.05) increases in FEV1 between QVA149 and both placebo and indacaterol (300 and 600 μg) were observed at all post-baseline time points on day 7 (figure 3A) and day 1 (figure 3B).
Forced expiratory volume in 1 s (FEV1) at all time points on (A) day 7 (B) day 1.
Trough FVC on days 1 and 7 (table 2) and the FVC at all post-baseline time points were significantly greater for QVA149A compared with both doses of indacaterol and placebo (p<0.05). Peak FEV1 on days 1 and 7 and standardised FEV1 AUC 5 min–4 h, and 5 min–23 h 45 min on day 1 and standardised FEV1 AUC 5 min–4 h, 5 min–12 h and 5 min–23 h 45 min on day 7 were also significantly greater in patients treated with QVA149 than in patients treated with placebo or indacaterol (table 2).
Bronchodilatory assessments (trough FVC, peak FEV1 and standardised FEV1 AUC and trough IC) at days 1 and 7 (mITT population)
The least squares means of IC and trough IC for QVA149 were significantly superior (p<0.05) to all other treatments from 4 h post-dose on day 1 onwards (trough least squares mean (SE) 2.18 (0.019)). The least squares means of IC and trough IC for QVA149 were also statistically superior (p<0.05) to all other treatments at all time points on day 7 (trough least squares mean (SE) 2.19 (0.021)) (table 2).
Rescue medication
The use of rescue medication was generally higher in patients receiving placebo compared with the active treatments. The number of puffs used was similar for the three active treatments. The number of patients using rescue medication was slightly higher in the QVA149-treated patients during day 1/2 (9.2%) compared with indacaterol 300 μg (7.8%) and indacaterol 600 μg (4.2%). However, during day 7/8, it was slightly lower in the QVA149 300/50 μg-treated patients (9.3%) compared with indacaterol 300 μg (11.4%) and indacaterol 600 μg (11.4%).
Safety
The majority of AE were mild in severity and were not suspected to be study drug related. The proportion of patients experiencing AE was similar between QVA149 and indacaterol 600 μg. Fewer patients experienced AE with indacaterol 300 μg and placebo (table 3). Two patients receiving QVA149 experienced serious AEs (fractured right arm and COPD exacerbation) and were discontinued from the study. None of the serious AEs were suspected of being related to the study drug. There were no clinically significant differences between the active treatments and placebo in terms of ECG evaluations, vital signs and laboratory evaluations. There was no difference between treatment groups with regard to the percentage of patients with notable QTc values (Fridericia's). No deaths were reported during the study.
Summary of most frequent AE (>2% population)
Discussion
This was the first study designed to assess the bronchodilatory effect of QVA149, a dual bronchodilator containing the LABA indacaterol and the LAMA NVA237 in patients with moderate to severe COPD. Following 7 days of treatment, once-daily QVA149 showed sustained 24-h bronchodilation that was significantly higher than placebo and indacaterol. The improvements in mean trough FEV1 exceeded the predefined minimal clinically important differences for QVA149 versus placebo and indacaterol. The onset of action of QVA149 was rapid as FEV1 was significantly higher compared with placebo and indacaterol at 5 min post-dose on days 1 and 7. QVA149 demonstrated significant improvements in all other spirometry results (FEV1 AUC, peak FEV1, IC and FVC) compared with indacaterol or placebo. Both QVA149 and indacaterol treatment resulted in a reduction in rescue medication use compared with placebo. Results presented here are consistent with those published previously indicating that LABA/LAMA combinations provide statistically significant and clinically relevant improvements in bronchodilation and COPD symptoms over each individual bronchodilator.3 6 8 10 13
A number of studies have assessed the efficacy and safety of tiotropium delivered in free combinations with formoterol. van Noord et al6 showed that a free combination of tiotropium plus formoterol given once daily in the morning was superior to twice-daily formoterol and once-daily tiotropium administered as monotherapies. Significant improvements in both day and night time FEV1 were obtained with combination compared with either monotherapy. In a longer-term study, it was shown that the addition of formoterol to tiotropium treatment conferred advantages in terms of early bronchodilator effect and lung function.10
The efficacy of indacaterol and NVA237 as once-daily therapies has been established.17–23 In our study, indacaterol produced similar and significant improvements in FEV1 compared with placebo; however, the addition of NVA237 to indacaterol produced substantial improvements in FEV1 over that achieved with either dose of indacaterol. The improvements in mean trough FEV1 obtained with QVA149 versus indacaterol 300 μg and 600 μg were 123 ml and 117 ml, respectively. This effect of QVA149 appears to be additive and greater than the addition of arformoterol (15 μg twice daily) to tiotropium (18 μg once daily), which resulted in a 70 ml improvement in trough FEV1 over either treatment alone.25 Once-daily indacaterol has been shown to provide clinically relevant 24-h bronchodilation that was as effective as tiotropium.22 Therefore, the significant improvements with QVA149 over indacaterol monotherapy are of considerable importance.
QVA149 was well tolerated and no safety signals were identified. The combined bronchodilator approach in this study did not appear to increase the burden of AE. These results are consistent with other studies using LABA/LAMA combinations,3 6–8 10 13 and for the monocomponents, indacaterol21–23 and NVA237.18 Future long-term studies will further assess the safety and tolerability profile of QVA149.
The results of this study also support the recommendations of current treatment guidelines that in patients with moderate, severe and very severe COPD, whose symptoms are not adequately controlled by maintenance monotherapy, bronchodilators of different classes can be combined. Such combinations may produce additional improvements in increasing the lung function and health status for equivalent or lesser side-effects.1 Most of the studies reported on LABA/LAMA combinations have focused on formoterol and salmeterol (both LABA, administered twice daily) and tiotropium (LAMA, once daily).3 6–14 However, these studies were conducted on free combinations of bronchodilators. By using QVA149, a combination of indacaterol and NVA237, our study adds further evidence with respect to the improvements in bronchodilation and lung function obtained by the addition of two 24-h bronchodilators in a single inhaler.
By combining two long-acting bronchodilators, once-daily QVA149 may help to simplify COPD management by providing bronchodilatory benefits over twice-daily bronchodilators. Moreover, it is more convenient to use a drug once daily than twice daily. This can potentially improve patient compliance, tolerability and safety, all of which may lead to better outcomes. Because patient adherence is a major obstacle to successful management of COPD, simplified once-daily dosing regimens may improve compliance26 27 and reduce the dose frequency to the minimum necessary in order to maintain disease control.28
In conclusion, QVA149 was effective, well tolerated and demonstrated rapid and sustained 24-h bronchodilation in patients with moderate to severe stable COPD. QVA149 may offer a simplified and more convenient dosing regimen compared with taking the two compounds as separate agents. Ongoing and future studies will further establish the efficacy and safety of QVA149 in patients with COPD.
Acknowledgments
The authors acknowledge and thank Shaik Asma Sultana, professional medical writer (Novartis), Mark Fedele (Novartis) and Gary Cotter (ACUMED UK) for assistance in the preparation of this manuscript.
References
Footnotes
Funding This study was funded entirely by Novartis.
Competing interests JAvN is involved in carrying out contract research for Boehringer Ingelheim, GSK and Chiesi. RB has received reimbursement for attending scientific conferences, and/or fees for speaking and/or consulting from Novartis. He also has similar relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Nycomed. CL is a member of the speaker's bureau for the following organisations: AstraZeneca, GlaxoSmithKline, Merck, Novartis, UCB Pharma, Sanofi-Aventis and Sepracor. He is also on the advisory boards at: GlaxoSmithKline, Schering-Plough, Alcon and Sepracor. CM, FJ, MD and TO are employees of Novartis.
Patient consent Obtained.
Ethics approval This study was conducted with the approval of the independent ethics committee orinstitutional review board for each participating study centre.
Provenance and peer review Not commissioned; externally peer reviewed.
↵* Breezhaler is a registered trademark of Novartis Pharma AG, Basel, Switzerland.