Article Text
Statistics from Altmetric.com
History
Behçet's disease is a chronic inflammatory disorder of unknown aetiology characterised by recurrent attacks. Although the triple symptom complex of oral and genital ulcerations with uveitis was reported by Hippocrates and other authors who attributed the symptom triad to major infections, Hulusi Behçet, a Turkish dermatologist, discarded the association with other illnesses and was the first to delineate the disease that now bears his name.1 Clinical manifestations additional to this triad were described later including involvement of the skin, joints, large vessels, lung, brain, gastrointestinal and genitourinary tracts (table 1).2-6It is now recognised as a multisystem disease with vasculitis as the main pathological finding.6-10
Diagnosis
Since Behçet's disease does not have pathognomonic symptoms or laboratory findings, the diagnosis is made on the basis of the criteria proposed by the International Study Group for Behçet's disease in 1990 (table 2).11 According to the criteria, recurrent oral ulceration must be present and at least two of the following: recurrent genital ulceration, eye lesions, skin lesions, or a positive pathergy test (development of a papule or pustule following a needle prick to the skin, as in table 2).
International Study Group criteria for the diagnosis of Behçet's disease11
Epidemiology
Although Behçet's disease has a worldwide distribution, most cases cluster along the ancient Silk Road which extends from far eastern Asia to the Mediterranean basin (table 3).12-18The highest prevalence rate was reported from Turkey as 80–370 per 100 000. The prevalence ranges from two to 30 cases per 100 000 in other Asian countries, with lower figures in Europe and the USA. The age of disease onset is usually in the second or third decade of life and the male to female ratio is reported to be almost equal. However, the disease runs a more severe course in men and in those with an onset before 25 years of age.7-10
Epidemiological data for Behçet's disease
Genetic association
Several studies have found a strong association between the risk of developing Behçet's disease and HLA-B51.19 ,20 The relative risk of the disease among carriers of this allele is up to 13.3 in Turkey compared with 1.3 in the USA.10 ,21 The association of HLA-B51 positivity with eye disease and a more severe clinical course is controversial.22
Pulmonary involvement
More than 200 cases of Behçet's disease with pulmonary involvement have been reported in the literature.23-50The pulmonary arteries are the second most common site of arterial involvement, preceded by the aorta. Aneurysms are more common than thrombosis.
Pulmonary artery aneurysms, arterial and venous thrombosis, pulmonary infarction, recurrent pneumonia, bronchiolitis obliterans organised pneumonia, and pleurisy are the main features of pulmonary involvement in Behçet's disease. The true prevalence of the pulmonary manifestations in Behçet's disease is unknown because no prospective study has evaluated all pulmonary symptoms in an unselected group of patients. The reported prevalence has ranged from 1% to 7.7%.2 ,40
PULMONARY ARTERY ANEURYSMS
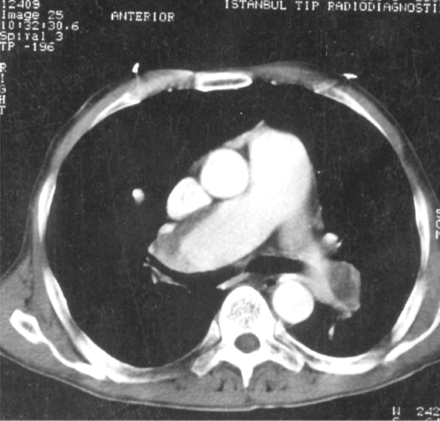
Pulmonary artery aneurysms affect mainly young men. Haemoptysis of varying degrees (up to 500 ml) is the most common and predominant symptom. Rupture of an aneurysm with erosion into a bronchus and the development of in situ thrombosis from active vasculitis have been suggested as explanations for the haemoptysis.23-50Sudden hilar enlargement or the appearance of polylobular and round opacities on the chest radiograph can represent pulmonary artery aneurysms (fig 1). When associated with an acute episode of haemoptysis they appear poorly marginated; otherwise, they have a distinct outline.40-50 Helical computed tomography is currently the method of choice for the diagnosis because it provides excellent vascular images with only a small quantity of contrast material.36-50 Aneurysms are seen as saccular or fusiform dilatations which show homogeneous contrast filling simultaneously with the pulmonary artery (figs 2, 3, 4). Pulmonary artery aneurysms are located most frequently in the right lower lobar arteries, followed by the right and left main pulmonary arteries.38 In this study the diameter of the aneurysms ranged from 1 to 7 cm and between two and seven aneurysms have been detected in the same patient. Magnetic resonance imaging is also helpful in the diagnosis of pulmonary artery aneurysms.51 ,52 Although no comparative studies are available, it is considered to be less sensitive than helical computed tomography in demonstrating small aneurysms. Digital substraction angiography has also been used in the diagnosis but it may be inadequate if aneurysms or vessels are completely thrombosed.37 ,38 ,48 ,52 Imaging techniques such as aortography, venography, and pulmonary angiography are no longer used as they carry a higher risk of complications. The frequency of such complications with digital substraction angiography is unknown. In one case report radionuclide angiography showed alterations in pulmonary artery blood flow as clearly as did subsequent contrast pulmonary angiography.53 Normal or aneurysmally dilated pulmonary arteries frequently become obliterated by large thrombi. On chest radiography this may result in hyperlucent areas of the lung supplied by these vessels. Computed tomographic scanning can show a mosaic pattern of variable attenuation reflecting non-homogeneous perfusion.38 Ventilation-perfusion lung scans show bilateral, well defined, mismatched areas.35 Although deep venous thrombosis of the lower extremities frequently accompanies pulmonary artery aneurysms, pulmonary thromboembolism is very rare in Behçet's disease because the thrombi in inflamed veins are strongly adherent.36
Chest radiograph of a patient with an aneurysm of the right pulmonary artery.(Courtesy of Dr T Ece).
Computed tomographic scan of a patient showing a thrombosed aneurysm of the right pulmonary artery. The fuzzy contour of the aneurysm wall and perianeurysmal consolidation are caused by an acute haemorrhage. (Courtesy of Dr A Tunaci).
Computed tomographic scan showing thrombosed pulmonary artery aneurysms. (Courtesy of Dr A Tunaci).
Computed tomographic scan showing multiple aneurysms of different branches of the pulmonary arteries bilaterally. (Courtesy of Dr A Tunaci).
PULMONARY PARENCHYMAL FINDINGS
Atelectasis, volume loss, wedge shaped, or linear shadows, ill defined, nodular or reticular opacities have been described in Behçet's disease with or without pulmonary artery aneurysms (fig5).23-48 These findings are generally accepted as foci of pulmonary haemorrhage and/or infarcts. However, the pathological correlation of the parenchymal opacities has only been documented in a few cases.26 ,37 ,54 A recent case report reported prominent clinical, radiological, and pathological findings of organising pneumonia associated with pulmonary artery aneurysms.54 Organising pneumonia may accompany various collagen vascular diseases including systemic lupus erythematosus and systemic vasculitides such as Wegener's granulomatosis.55 ,56 Patients with secondary organising pneumonia have a worse prognosis than cryptogenic or primary cases.57 In another patient with Behçet's disease and peripheral non-segmental pulmonary infiltrates, eosinophilic pneumonia was found on transbronchial biopsy.37
Computed tomographic scan showing a focal non-segmental infiltrate in the left subpleural region in a patient with Behçet's disease. (Courtesy of Dr A Tunaci).
OTHER THORACIC MANIFESTATIONS OF BEHçET'S DISEASE
Involvement of major veins including occlusion of the superior vena cava is a more prevalent finding than arteritis. Thrombosis of the innominate and subclavian veins may accompany superior vena caval occlusion.58-61 Magnetic resonance imaging is the suggested diagnostic method. Pseudoaneurysms of the aortic arch as well as the subclavian and coronary arteries have been described in Behçet's disease.62 ,63 Mediastinal mass, mediastinitis, chyloptysis, and pleurisy are other associated conditions.32 ,37 ,40 ,64 Pleural effusion may result from vasculitis of the pleura or thrombosis of the superior vena cava.37
Pathology
The central feature of the histopathology of Behçet's disease is systemic vasculitis and perivascular inflammatory infiltrates.5 ,6 The vasculitis can involve large, medium, and small vessels of both the arterial and venous circulation. Inflammatory infiltrates may be granulocytic, mononuclear, or mixed (fig 6). There is a tendency to thrombus formation with thrombi in the lumen of vessels showing features of inflammation and focal areas of lymphocytes. Pathologically, the pulmonary artery aneurysms have perivascular infiltrates around the vasa vasorum, marked intimal thickening with degenerative changes in the elastic lamina, thrombotic occlusion, and recanalisation as well as fresh thrombi.32 ,54
Photomicrograph of the histopathology of a pulmonary vessel from a pneumonectomy sample from a patient suffering major haemoptysis due to Behcet's disease showing a neutrophilic vasculitis. Stain: H&E, ×310. (Courtesy of Dr D Yilmazbayhan).
Natural history and prognosis
The natural history of Behçet's disease is one of exacerbations and remissions. Male sex and young age of onset are markers of a more severe prognosis. Pulmonary artery aneurysm formation has a very poor prognosis and is one of the leading causes of death in Behçet's disease; 30% of patients with this condition die within 2 years.32 ,36 ,40 Mean survival after the onset of haemoptysis was reported to be about 10 months in one study of patients with Behçet's disease and pulmonary artery aneurysms.36A more recent follow up study of computed tomographic findings in 13 patients receiving immunosuppressant treatment showed complete disappearance or regression of pulmonary artery aneurysms during 3–42 (mean 21) months of treatment.38 Disappearance and regression of the aneurysm were preceded by thrombus formation. After treatment the thrombi regressed and pulmonary artery aneurysms disappeared. Massive bleeding has been reported in patients receiving immunosuppressant treatment, although a partial remission was achieved.36 ,38 ,54
Management
IMMUNOSUPPRESSANT TREATMENT
Empirical anti-inflammatory and/or immunosuppressive drugs tailored to the severity of the disease remain the mainstay of treatment.65-67 A combination of cyclophosphamide and methylprednisolone is used most frequently for patients with pulmonary artery aneurysms,7 ,8 ,67 although no controlled trial has assessed the efficacy of this combination. For patients with pulmonary artery aneurysms we give cyclophosphamide 1000 mg monthly as intravenous pulses or 2 mg/kg/day orally with oral methylprednisolone 1 mg/kg. For patients with severe haemoptysis we start with intravenous pulses of methylprednisolone 500–1000 mg for 3 days together with pulsed cyclophosphamide.7 ,8 ,64 The prednisolone dose is then tapered depending on the clinical response, while the cyclophosphamide regimen is continued for at least 1 year after complete remission when it is frequently switched to azathioprine.35 ,36 ,38 ,40 Cylosporin combined with coumarin was reported to be successful in a patient with a single pulmonary artery aneurysm34 and FK506 was used with good results in a patient with pulmonary infiltrates.68 Double blind controlled trials are needed to assess the efficacy and long term effects of currently used and new immunosuppressant drugs for eye lesions and/or life threatening complications in Behçet's disease.
THROMBOLYTIC AND ANTICOAGULANT TREATMENT
Haemoptysis in Behçet's disease frequently leads to the misdiagnosis of pulmonary thromboembolism due to the frequent presence of a peripheral deep vein thrombosis and an abnormal ventilation-perfusion scan. Anticoagulation carries significant risks for patients with pulmonary artery aneurysms and must be used cautiously and only after systemic immunosuppressant treatment has been given.40 If thrombi are not extensive, antiplatelet treatment with, for example, low dose aspirin, is probably sufficient.35 ,40 Thrombolytic treatment with urokinase was tried in one patient with a thrombosed pulmonary artery aneurysm69 and streptokinase was given to a patient with superior vena cava syndrome.60 There was no evidence of new thrombotic episodes over the subsequent 2 year follow up period.69 Both patients were also receiving immunosuppressive treatment so the risks and efficacy of thrombolytic treatment is difficult to assess. There are no controlled studies of anticoagulants or antiplatelet aggregation therapy and there is a lack of consensus on their use.
Clinical trials are needed to address the place of these drugs in the management of thrombotic disease in these patients.
EMBOLISATION
Embolisation of a pulmonary artery aneurysm was attempted in one patient with massive bleeding.70 The size and number of aneurysms, the presence of superior or inferior vena caval occlusion, and the potential complication of severe bleeding are the main limitations to the use of embolisation in Behçet's disease.
SURGERY
In cases of massive haemoptysis urgent surgical resection may be necessary. The main problem facing the vascular surgeon is the 25% incidence of recurrent anastomotic aneurysms after both inlay graft repair and patching.71-74 False aneurysms and arteriovenous fistulae are also common at sites of previous iatrogenic trauma. Perioperative steroid cover has been suggested to reduce the risk of complications.
In conclusion, therefore, the mainstay of treatment in Behcet's disease is immunosuppressant therapy as in other severe vasculitides. Other treatment modalities should be used only in combination with this therapy and as palliative measures for specific complications.
Pathogenesis
The aetiology of Behçet's disease is unknown. Clinical observations and laboratory investigations support the concept that immunological mechanisms induced by microbial pathogens occur in genetically susceptible individuals.7 ,75
Although the majority of patients with Behçet's disease have no family history, a familial clustering can occur. Recent studies have shown that the risk of developing Behçet's disease by siblings of index patients is increased compared with the general population. Sibling risk ratio (λs, the ratio of sibling prevalence to population prevalence of disease) of Behçet's disease was reported to be 11.4–52.5 in Turkey, supporting the concept of an important genetic influence.76
The association of HLA-B51 with Behçet's disease is the strongest finding supporting the contribution of genetics to the pathogenesis of the disease.19 ,20 It remains to be clarified whether HLA-B51 has a direct role in the pathogenesis or whether this association reflects linkage disequilibrium with a disease associated susceptibility gene located near the HLA-B locus. Microsatellite polymorphism studies have identified a strong association of Behçet's disease with MHC class I Chain related gene A (MICA), which is located 46 kb centromeric to HLA-B.77 However, HLA-B51 still shows the strongest association with Behçet's disease among the various polymorphisms in and around the critical region between HLA-B and MICA, and it is difficult to assess the individual supplementary effects of MICA or other neighbouring genes on HLA-B51 carrying haplotype.78 ,79 On the other hand, family studies indicate that the contribution of the HLA-B locus (HLA-B51) to the overall genetic susceptibility to Behçet's disease is less than 20%.80 Identification of other susceptibility loci is required.
Non-specific hyperreactivity is an important feature of Behçet's disease. The classical example is the skin pathergy reaction in which a papule or pustule occurs following a simple needle prick to the skin, similar to those appearing spontaneously in the disease.81This increased responsiveness to minor trauma or other stimuli is not unique to the skin, and the phenomenon can be seen at other sites or even at the cellular level as an upregulated inflammatory response.82 Increased expression of several cytokines from lymphocytes and monocytes has been reported.83-87Oversecretion of the Th1 type proinflammatory cytokines such as interleukin 2 (IL-2) and interferon γ (IFNγ) is prominent, especially during the active phase of the disease.86 ,87Increased secretion of IL-1, IL-6, tumour necrosis factor α (TNFα) and IL-8 from monocytes following lipopolysaccharide stimulation compared with controls can also be observed, even in patients with inactive disease, and immunosuppressant drugs might not suppress this lymphocyte hypersensitivity as demonstrated by increased IFNγ production in response to bacterial antigens in vitro.82 ,83
Activation of neutrophils with increased chemotaxis and superoxide generation has long been suggested as the main pathogenetic mechanism in Behçet's disease.75 ,88 ,89 Increased production of some cytokines such as IL-8, TNFα, and IL-1 from lymphomononuclear cells, neutrophils, and/or endothelial cells may have a regulatory role in neutrophil function and might be responsible for this primed state. A similar neutrophil hyperactivity after fMLP stimulation was the only abnormal finding in HLA-B51 transgenic mice, and enhanced superoxide generation has been observed in HLA-B51 positive healthy controls.89 ,90 The relationship between HLA-B51 and neutrophil function remains to be clarified.
Recent studies point to a central role for the T cell mediated immune response in the pathogenesis of Behçet's disease.82 ,84-87 ,91 ,92 Oligoclonal T cell expansions correlate with clinically active disease, supporting the view that an antigen driven immune response contributes to its immunopathogenesis.84 ,91 Several microbial agents, especially herpes simplex virus and certain strains of streptococci, have been claimed to induce the manifestations of Behçet's disease. A unifying hypothesis suggests that certain epitopes of microbial heat shock proteins (hsp) are putative antigens, triggering a specific immune response and producing a “cross reacting” inflammatory reaction.92 An increased T cell response has been shown against four peptides derived from 65 kD mycobacterial hsp and their human homologues in British, Japanese, and Turkish patients with Behçet's disease.84 ,92 ,93 A subset of γδ+ T cells is increased in Behçet's disease and may have a regulatory role in response to these peptides.93-95 The hsp peptides cause uveitis in rats, and an association between a T cell proliferative response to one of the human 60 kd hsp peptides and the presence of ocular lesions has been reported in patients with Behçet's disease.84 ,96
Hormonal factors may also have a place in the pathogenesis of Behçet's disease. Although the number of men and women affected is similar in many series, men tend to have more severe disease.97 ,98 Acneiform skin lesions of Behçet's disease cannot be differentiated clinically or histologically from ordinary acne seen in cases of excess androgen.99 Detailed hormonal studies are needed to understand the contribution of sex hormones to the expression and severity of Behçet's disease.
Prospective studies
New diagnostic methods are helping to show the full clinical picture of Behçet's disease and to document some of the rarer manifestations such as parenchymal lung involvement and gastrointestinal disease.
Whole genome screening in multicase Behçet's disease families and association studies for candidate genes in cases and controls would help to identify other susceptibility genes. Elucidation of pathogenetic mechanisms may help to develop better therapeutic modalities—for example, the studies suggesting an important role for hsp derived peptides in immunological activation in Behçet's disease are being followed by experimental studies to try to induce tolerance using hsp peptides.
References
1st Asia Pacific Forum on Quality Improvement in Health Care Three day conference Wednesday 19 to Friday 21 September 2001 Sydney, Australia
We are delighted to announce this forthcoming conference in Sydney. Authors are invited to submit papers (call for papers closes on Friday 6 April), and delegate enquiries are welcome.
The themes of the Forum are:
Improving patient safety
Leadership for improvement
Consumers driving change
Building capacity for change: measurement, education and human resources
The context: incentives and barriers for change
Improving health systems
The evidence and scientific basis for quality improvement.
Presented to you by the BMJ Publishing Group (London, UK) and Institute for Healthcare Improvement (Boston, USA), with the support of the the Commonwealth Department of Health and Aged Care (Australia), Safety and Quality Council (Australia), NSW Health (Australia), and Ministry of Health (New Zealand).
For more information contact:quality{at}bma.org.uk or fax +44 (0)20 7383 6869