Key Points
-
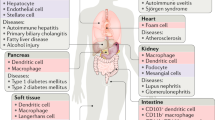
Apoptotic cells are rapidly removed by tissue-resident professional phagocytes and/or by neighbouring non-professional phagocytes under homeostatic conditions.
-
The prompt clearance of apoptotic cells involves molecular steps that include the recruitment of phagocytes towards apoptotic cells through 'find-me' signals and the recognition of 'eat-me' signals on apoptotic cells that trigger engulfment.
-
Apoptotic cell clearance under physiological conditions is generally anti-inflammatory and immunologically silent.
-
Defects in apoptotic cell removal are associated with the initiation and progression of a number of pathological conditions, including inflammation and autoimmunity.
-
The process of apoptotic cell clearance can be manipulated potentially by pharmacological means to treat a variety of human diseases.
Abstract
The prompt removal of apoptotic cells by phagocytes is important for maintaining tissue homeostasis. The molecular and cellular events that underpin apoptotic cell recognition and uptake, and the subsequent biological responses, are increasingly better defined. The detection and disposal of apoptotic cells generally promote an anti-inflammatory response at the tissue level, as well as immunological tolerance. Consequently, defects in apoptotic cell clearance have been linked with various inflammatory diseases and autoimmunity. Conversely, under certain conditions, such as the killing of tumour cells by specific cell-death inducers, the recognition of apoptotic tumour cells can promote an immunogenic response and antitumour immunity. Here, we review the current understanding of the complex process of apoptotic cell clearance in physiology and pathology, and discuss how this knowledge could be harnessed for new therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ravichandran, K. S. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J. Exp. Med. 207, 1807–1817 (2010). This review offers a discussion of PtdSer recognition and the molecular mechanisms underpinning apoptotic cell clearance.
Wood, W. et al. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development 127, 5245–5252 (2000).
Parnaik, R., Raff, M. C. & Scholes, J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr. Biol. 10, 857–860 (2000).
Monks, J., Smith-Steinhart, C., Kruk, E. R., Fadok, V. A. & Henson, P. M. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol. Reprod. 78, 586–594 (2008).
Juncadella, I. J. et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 493, 547–551 (2013).
Cinti, S. et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 (2005).
Sebbagh, M. et al. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nature Cell Biol. 3, 346–352 (2001).
Coleman, M. L. et al. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nature Cell Biol. 3, 339–345 (2001).
Hochreiter-Hufford, A. E. et al. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 497, 263–267 (2013). This study describes an unexpected role for apoptotic cells in promoting myoblast fusion.
Levkau, B., Herren, B., Koyama, H., Ross, R. & Raines, E. W. Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J. Exp. Med. 187, 579–586 (1998).
Kook, S. et al. Caspase-mediated cleavage of p130cas in etoposide-induced apoptotic Rat-1 cells. Mol. Biol. Cell 11, 929–939 (2000).
Schmeiser, K. & Grand, R. J. The fate of E- and P-cadherin during the early stages of apoptosis. Cell Death Differ. 6, 377–386 (1999).
Brancolini, C., Lazarevic, D., Rodriguez, J. & Schneider, C. Dismantling cell-cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of β-catenin. J. Cell Biol. 139, 759–771 (1997).
Rosenblatt, J., Raff, M. C. & Cramer, L. P. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 11, 1847–1857 (2001). This study describes the extrusion of apoptotic epithelial cells by neighbouring cells as a mechanism to maintain the epithelial barrier.
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009). An important study that established the role of nucleotides as chemotactic signals released by apoptotic cells to attract phagocytes to promote cell clearance.
Chekeni, F. B. et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature 467, 863–867 (2010).
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nature Rev. Cancer 12, 860–875 (2012).
Lauber, K. et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113, 717–730 (2003).
Gude, D. R. et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J. 22, 2629–2638 (2008).
Segundo, C. et al. Surface molecule loss and bleb formation by human germinal center B cells undergoing apoptosis: role of apoptotic blebs in monocyte chemotaxis. Blood 94, 1012–1020 (1999).
Torr, E. E. et al. Apoptotic cell-derived ICAM-3 promotes both macrophage chemoattraction to and tethering of apoptotic cells. Cell Death Differ. 19, 671–679 (2012).
Truman, L. A. et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 112, 5026–5036 (2008). This study shows that certain find-me signals can be released from apoptotic cells through microparticles.
Bournazou, I. et al. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J. Clin. Invest. 119, 20–32 (2009). The first demonstration that apoptotic cells can actively release keep-out signals to inhibit the recruitment of neutrophils.
Bournazou, I., Mackenzie, K. J., Duffin, R., Rossi, A. G. & Gregory, C. D. Inhibition of eosinophil migration by lactoferrin. Immunol. Cell Biol. 88, 220–223 (2010).
Fadok, V. A. et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216 (1992).
Verhoven, B., Schlegel, R. A. & Williamson, P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J. Exp. Med. 182, 1597–1601 (1995).
Bratton, D. L. et al. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J. Biol. Chem. 272, 26159–26165 (1997).
Suzuki, J., Denning, D. P., Imanishi, E., Horvitz, H. R. & Nagata, S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341, 403–406 (2013). This report suggests that Xk-family proteins could be involved in regulating the exposure of PtdSer during apoptosis.
Borisenko, G. G. et al. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells—existence of a threshold. Arch. Biochem. Biophys. 413, 41–52 (2003).
Park, D. et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/ Rac module. Nature 450, 430–434 (2007).
Park, S. Y. et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 15, 192–201 (2008).
Park, S. Y., Kim, S. Y., Jung, M. Y., Bae, D. J. & Kim, I. S. Epidermal growth factor-like domain repeat of stabilin-2 recognizes phosphatidylserine during cell corpse clearance. Mol. Cell. Biol. 28, 5288–5298 (2008).
Kobayashi, N. et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27, 927–940 (2007).
Miyanishi, M. et al. Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439 (2007).
Nakayama, M. et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 113, 3821–3830 (2009).
Lee, S. J., So, I. S., Park, S. Y. & Kim, I. S. Thymosin beta4 is involved in stabilin-2-mediated apoptotic cell engulfment. FEBS Lett. 582, 2161–2166 (2008).
Park, S. Y. et al. Requirement of adaptor protein GULP during stabilin-2-mediated cell corpse engulfment. J. Biol. Chem. 283, 10593–10600 (2008).
Toda, S., Hanayama, R. & Nagata, S. Two-step engulfment of apoptotic cells. Mol. Cell. Biol. 32, 118–125 (2012).
Anderson, H. A. et al. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nature Immunol. 4, 87–91 (2003).
Hanayama, R. et al. Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187 (2002).
Ishimoto, Y., Ohashi, K., Mizuno, K. & Nakano, T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J. Biochem. 127, 411–417 (2000).
Gardai, S. J. et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123, 321–334 (2005).
Garg, A. D. et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 31, 1062–1079 (2012).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Med. 13, 54–61 (2007). An important study demonstrating that the exposure of CRT on apoptotic cancer cells could be crucial for the generation of antitumour immunity.
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013).
Segawa, K., Suzuki, J. & Nagata, S. Constitutive exposure of phosphatidylserine on viable cells. Proc. Natl Acad. Sci. USA 108, 19246–19251 (2011).
Oldenborg, P. A. et al. Role of CD47 as a marker of self on red blood cells. Science 288, 2051–2054 (2000). An early study that describes CD47 as a don't eat-me signal.
Okazawa, H. et al. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J. Immunol. 174, 2004–2011 (2005).
Elward, K. et al. CD46 plays a key role in tailoring innate immune recognition of apoptotic and necrotic cells. J. Biol. Chem. 280, 36342–36354 (2005).
Li, F. et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci. Signal. 3, ra13 (2010).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Serhan, C. N. et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21, 325–332 (2007).
Zemans, R. L. et al. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc. Natl Acad. Sci. USA 108, 15990–15995 (2011).
Farnworth, S. L. et al. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am. J. Pathol. 172, 395–405 (2008).
Persson, C. G. & Uller, L. Resolution of cell-mediated airways diseases. Respir. Res. 11, 75 (2010).
Beauvillain, C. et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood 117, 1196–1204 (2011).
Savill, J. S. et al. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83, 865–875 (1989). The first recognition that neutrophil apoptosis governs subsequent phagocytosis by macrophages and that this represents a critical process in the resolution of inflammation.
Haslett, C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Respiratory Crit. Care Med. 160, S5–S11 (1999).
Sexton, D. W., Al-Rabia, M., Blaylock, M. G. & Walsh, G. M. Phagocytosis of apoptotic eosinophils but not neutrophils by bronchial epithelial cells. Clin. Exp. Allergy 34, 1514–1524 (2004).
Watson, R. W., Redmond, H. P., Wang, J. H., Condron, C. & Bouchier-Hayes, D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J. Immunol. 156, 3986–3992 (1996).
Koedel, U. et al. Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog. 5, e1000461 (2009).
El Kebir, D., Gjorstrup, P. & Filep, J. G. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl Acad. Sci. USA 109, 14983–14988 (2012).
Rupp, J. et al. Chlamydia pneumonia ehides inside apoptotic neutrophils to silently infect and propagate in macrophages. PLoS ONE 4, e6020 (2009).
Handa, Y. et al. Shigella IpgB1 promotes bacterial entry through the ELMO-Dock180 machinery. Nature Cell Biol. 9, 121–128 (2007).
Hodge, S., Hodge, G., Scicchitano, R., Reynolds, P. N. & Holmes, M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 81, 289–296 (2003).
Morimoto, K., Janssen, W. J. & Terada, M. Defective efferocytosis by alveolar macrophages in IPF patients. Respir. Med. 106, 1800–1803 (2012).
Vandivier, R. W. et al. Impaired clearance of apoptotic cells from cystic fibrosis airways. Chest 121, 89S (2002).
McPhillips, K. et al. TNF-α inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J. Immunol. 178, 8117–8126 (2007).
Nakaya, M., Tanaka, M., Okabe, Y., Hanayama, R. & Nagata, S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J. Biol. Chem. 281, 8836–8842 (2006).
Tosello-Trampont, A. C., Nakada-Tsukui, K. & Ravichandran, K. S. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J. Biol. Chem. 278, 49911–49919 (2003).
Moon, C., Lee, Y. J., Park, H. J., Chong, Y. H. & Kang, J. L. N-acetylcysteine inhibits RhoA and promotes apoptotic cell clearance during intense lung inflammation. Am. J. Respir. Crit. Care Med. 181, 374–387 (2009).
Cepkova, M. & Matthay, M. A. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J. Intensive Care Med. 21, 119–143 (2006).
Huynh, M. L. et al. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am. J. Respir. Crit. Care Med. 172, 972–979 (2005).
Fitzpatrick, A. M., Holguin, F., Teague, W. G. & Brown, L. A. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J. Allergy Clin. Immunol. 121, 1372–1378, 1378 e1–3 (2008).
Meagher, L. C., Cousin, J. M., Seckl, J. R. & Haslett, C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J. Immunol. 156, 4422–4428 (1996).
Liu, Y. et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 162, 3639–3646 (1999). This study demonstrated for the first time that the commonly used anti-inflammatory glucocorticoids enhance macrophage phagocytosis of apoptotic cells.
McColl, A. et al. Glucocorticoids induce protein S-dependent phagocytosis of apoptotic neutrophils by human macrophages. J. Immunol. 183, 2167–2175 (2009).
Woolley, K. L. et al. Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am. J. Respir. Crit. Care Med. 154, 237–243 (1996).
Vago, J. P. et al. Annexin A1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. J. Leukocyte Biol. 92, 249–258 (2012).
Marwick, J. A. et al. Oxygen levels determine the ability of glucocorticoids to influence neutrophil survival in inflammatory environments. J. Leukocyte Biol. 94, 1285–1292 (2013).
Ley, K., Miller, Y. I. & Hedrick, C. C. Monocyte and macrophage dynamics during atherogenesis. Arterioscler. Thromb. Vasc. Biol. 31, 1506–1516 (2011).
Tabas, I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb. Vasc. Biol. 25, 2255–2264 (2005).
Schrijvers, D. M., De Meyer, G. R., Kockx, M. M., Herman, A. G. & Martinet, W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb. Vasc. Biol. 25, 1256–1261 (2005).
Schrijvers, D. M., De Meyer, G. R., Herman, A. G. & Martinet, W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc. Res. 73, 470–480 (2007).
Ait-Oufella, H. et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 115, 2168–2177 (2007).
Tabas, I. Macrophage death and defective inflammation resolution in atherosclerosis. Nature Rev. Immunol. 10, 36–46 (2010).
Thorp, E., Cui, D., Schrijvers, D. M., Kuriakose, G. & Tabas, I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of Apoe−/− mice. Arterioscler. Thromb. Vasc. Biol. 28, 1421–1428 (2008).
Bhatia, V. K. et al. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am. J. Pathol. 170, 416–426 (2007).
Deftereos, S. et al. Association of soluble tumour necrosis factor-related apoptosis-inducing ligand levels with coronary plaque burden and composition. Heart 98, 214–218 (2012).
Miller, Y. I. et al. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 278, 1561–1568 (2003).
Loirand, G., Guerin, P. & Pacaud, P. Rho kinases in cardiovascular physiology and pathophysiology. Circ. Res. 98, 322–334 (2006).
Ridker, P. M. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation 108, 2292–2297 (2003).
Morimoto, K. et al. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J. Immunol. 176, 7657–7665 (2006).
Wu, D. J. et al. Effects of fasudil on early atherosclerotic plaque formation and established lesion progression in apolipoprotein E-knockout mice. Atherosclerosis 207, 68–73 (2009).
Grommes, J. et al. Simvastatin reduces endotoxin-induced acute lung injury by decreasing neutrophil recruitment and radical formation. PLoS ONE 7, e38917 (2012).
Jiang, C. et al. Fasudil, a rho-kinase inhibitor, attenuates bleomycin-induced pulmonary fibrosis in mice. Int. J. Mol. Sci. 13, 8293–8307 (2012).
Leung, B. P. et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J. Immunol. 170, 1524–1530 (2003).
Tabas, I., Williams, K. J. & Boren, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116, 1832–1844 (2007).
Li, A. C. & Glass, C. K. The macrophage foam cell as a target for therapeutic intervention. Nature Med. 8, 1235–1242 (2002).
Cuchel, M. & Rader, D. J. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation 113, 2548–2555 (2006).
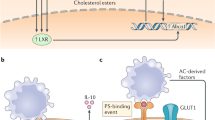
Kiss, R. S., Elliott, M. R., Ma, Z., Marcel, Y. L. & Ravichandran, K. S. Apoptotic cells induce a phosphatidylserine-dependent homeostatic response from phagocytes. Curr. Biol. 16, 2252–2258 (2006).
Lee, J. Y. & Parks, J. S. ATP-binding cassette transporter AI and its role in HDL formation. Curr. Opin. Lipidol. 16, 19–25 (2005).
Oram, J. F. & Heinecke, J. W. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85, 1343–1372 (2005).
Van Eck, M. et al. Macrophage ATP-binding cassette transporter A1 overexpression inhibits atherosclerotic lesion progression in low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vascular Biol. 26, 929–934 (2006).
Aiello, R. J. et al. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler. Thromb. Vascular Biol. 22, 630–637 (2002).
Tang, C., Liu, Y., Kessler, P. S., Vaughan, A. M. & Oram, J. F. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J. Biol. Chem. 284, 32336–32343 (2009).
Zhu, X. et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 51, 3196–3206 (2010).
A.-Gonzalez, N. et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31, 245–258 (2009).
Vucic, E. et al. Regression of inflammation in atherosclerosis by the LXR agonist R211945: a noninvasive assessment and comparison with atorvastatin. JACC Cardiovasc. Imag. 5, 819–828 (2012).
Fernandez-Boyanapalli, R. et al. PPARγ activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease. Blood 116, 4512–4522 (2010).
Nissen, S. E. et al. Comparison of pioglitazone versus glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 299, 1561–1573 (2008).
Rumore, P. M. & Steinman, C. R. Endogenous circulating DNA in systemic lupus erythematosus. Occurrence as multimeric complexes bound to histone. J. Clin. Invest. 86, 69–74 (1990).
Perniok, A., Wedekind, F., Herrmann, M., Specker, C. & Schneider, M. High levels of circulating early apoptic peripheral blood mononuclear cells in systemic lupus erythematosus. Lupus 7, 113–118 (1998).
Baumann, I. et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 46, 191–201 (2002).
Herrmann, M. et al. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 41, 1241–1250 (1998).
Hanayama, R. et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304, 1147–1150 (2004).
Hu, C. Y. et al. Genetic polymorphism in milk fat globule-EGF factor 8 (MFG-E8) is associated with systemic lupus erythematosus in human. Lupus 18, 676–681 (2009).
Yamaguchi, H. et al. Aberrant splicing of the milk fat globule-EGF factor 8 (MFG-E8) gene in human systemic lupus erythematosus. Eur. J. Immunol. 40, 1778–1785 (2010).
Botto, M. & Walport, M. J. C1q, autoimmunity and apoptosis. Immunobiology 205, 395–406 (2002).
Botto, M. et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature Genet. 19, 56–59 (1998).
Devitt, A. et al. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14−/− mice. J. Cell Biol. 167, 1161–1170 (2004).
Stuart, L. M., Takahashi, K., Shi, L., Savill, J. & Ezekowitz, R. A. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J. Immunol. 174, 3220–3226 (2005).
Kenyon, K. D. et al. IgG autoantibodies against deposited C3 inhibit macrophage-mediated apoptotic cell engulfment in systemic autoimmunity. J. Immunol. 187, 2101–2111 (2011).
Hurst, N. P., Nuki, G. & Wallington, T. Functional defects of monocyte C3b receptor-mediated phagocytosis in rheumatoid arthritis (RA): evidence for an association with the appearance of a circulating population of non-specific esterase-negative mononuclear phagocytes. Ann. Rheum. Dis. 42, 487–493 (1983).
Friggeri, A. et al. Extracellular histones inhibit efferocytosis. Mol. Med. 18, 825–833 (2012).
Friggeri, A. et al. HMGB1 inhibits macrophage activity in efferocytosis through binding to the αvβ3-integrin. Am. J. Physiol. Cell Physiol. 299, 1267–1276 (2010).
van den Brand, B. T. et al. Therapeutic efficacy of Tyro3, Axl, and Mer tyrosine kinase agonists in collagen-induced arthritis. Arthritis Rheum. 65, 671–680 (2013).
Park, M. C., Kwon, Y. J., Chung, S. J., Park, Y. B. & Lee, S. K. Liver X receptor agonist prevents the evolution of collagen-induced arthritis in mice. Rheumatol. (Oxford) 49, 882–890 (2010).
Tomita, T., Kakiuchi, Y. & Tsao, P. S. THR0921, a novel peroxisome proliferator-activated receptor gamma agonist, reduces the severity of collagen-induced arthritis. Arthritis Res. Ther. 8, R7 (2006).
Morelli, A. E. & Larregina, A. T. Apoptotic cell-based therapies against transplant rejection: role of recipient's dendritic cells. Apoptosis 15, 1083–1097 (2010). An excellent review that summarizes the beneficial properties of apoptotic cells in tissue transplantation.
Bittencourt, M. C. et al. Intravenous injection of apoptotic leukocytes enhances bone marrow engraftment across major histocompatibility barriers. Blood 98, 224–230 (2001).
Sun, E. et al. Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell Death Differ. 11, 1258–1264 (2004).
Wang, Z. et al. Use of the inhibitory effect of apoptotic cells on dendritic cells for graft survival via T-cell deletion and regulatory T cells. Am. J. Transplant 6, 1297–1311 (2006).
Kleinclauss, F. et al. Intravenous apoptotic spleen cell infusion induces a TGF-β-dependent regulatory T-cell expansion. Cell Death Differ. 13, 41–52 (2006).
Wang, Z. et al. In situ-targeting of dendritic cells with donor-derived apoptotic cells restrains indirect allorecognition and ameliorates allograft vasculopathy. PLoS ONE 4, e4940 (2009).
Gregory, C. D. & Pound, J. D. Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J. Pathol. 223, 177–194 (2011).
Bondanza, A. et al. Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo. J. Exp. Med. 200, 1157–1165 (2004).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Condeelis, J. & Pollard, J. W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263–266 (2006).
Casares, N. et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 202, 1691–1701 (2005).
Spisek, R. et al. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood 109, 4839–4845 (2007).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Med. 13, 1050–1059 (2007).
Jaiswal, S. et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138, 271–285 (2009).
Chao, M. P. et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142, 699–713 (2010). This study provides the first evidence that targeting CD47 in combination with another antibody therapy (rituximab) could be effective in treating non-Hodgkin lymphoma in animal models.
Willingham, S. B. et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl Acad. Sci. USA 109, 6662–6667 (2012).
Tseng, D. et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl Acad. Sci. USA 110, 11103–11108 (2013).
Weiskopf, K. et al. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science 341, 88–91 (2013).
Vandenabeele, P., Galluzzi, L., Vanden Berghe, T. & Kroemer, G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature Rev. Mol. Cell Biol. 11, 700–714 (2010).
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nature Rev. Microbiol. 7, 99–109 (2009).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Ueki, S. et al. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood 121, 2074–2083 (2013).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Kono, H. & Rock, K. L. How dying cells alert the immune system to danger. Nature Rev. Immunol. 8, 279–289 (2008).
Bonilla, W. V. et al. The alarmin interleukin-33 drives protective antiviral CD8+ T cell responses. Science 335, 984–989 (2012).
Chen, G. Y., Tang, J., Zheng, P. & Liu, Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 323, 1722–1725 (2009).
Poon, I. K., Hulett, M. D. & Parish, C. R. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 17, 381–397 (2010). This comprehensive review article summarizes the molecular mechanisms of clearance for membrane-permeabilized cells.
Sancho, D. et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 458, 899–903 (2009).
Zhang, J. G. et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity 36, 646–657 (2012).
Serhan, C. N. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol. 177, 1576–1591 (2010).
Dalli, J. & Serhan, C. N. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–e72 (2012).
Levy, B. D. et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4). Nature Med. 8, 1018–1023 (2002).
El Kebir, D. et al. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am. J. Respir. Crit. Care Med. 180, 311–319 (2009).
Arita, M. et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl Acad. Sci. USA 102, 7671–7676 (2005).
Chiang, N. et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 (2012).
Fredman, G. et al. Impaired phagocytosis in localized aggressive periodontitis: rescue by Resolvin E1. PLoS ONE 6, e24422 (2011).
Frasch, S. C. et al. Neutrophils regulate tissue neutrophilia in inflammation via the oxidant modified lipid lysophosphatidylserine. J. Biol. Chem. 5, 5 (2013).
Frasch, S. C. & Bratton, D. L. Emerging roles for lysophosphatidylserine in resolution of inflammation. Prog. Lipid Res. 51, 199–207 (2012).
Fernandez-Boyanapalli, R. F. et al. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood 113, 2047–2055 (2009).
Uderhardt, S. et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity 36, 834–846 (2012).
Szodoray, P., Papp, G., Nakken, B., Harangi, M. & Zeher, M. The molecular and clinical rationale of extracorporeal photochemotherapy in autoimmune diseases, malignancies and transplantation. Autoimmun Rev. 9, 459–464 (2010).
Korns, D., Frasch, S. C., Fernandez-Boyanapalli, R., Henson, P. M. & Bratton, D. L. Modulation of macrophage efferocytosis in inflammation. Front. Immunol. 2, 57 (2011).
Perruche, S., Saas, P. & Chen, W. Apoptotic cell-mediated suppression of streptococcal cell wall-induced arthritis is associated with alteration of macrophage function and local regulatory T-cell increase: a potential cell-based therapy? Arthritis Res. Ther. 11, R104 (2009).
Michlewska, S., Dransfield, I., Megson, I. L. & Rossi, A. G. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-α. FASEB J. 23, 844–854 (2009).
Huynh, M. L., Fadok, V. A. & Henson, P. M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Invest. 109, 41–50 (2002).
Lee, Y. J. et al. Apoptotic cell instillation after bleomycin attenuates lung injury through hepatocyte growth factor induction. Eur. Respir. J. 40, 424–435 (2012).
Ren, Y. et al. Apoptotic cells protect mice against lipopolysaccharide-induced shock. J. Immunol. 180, 4978–4985 (2008). This study demonstrated that delivery of exogenously produced apoptotic cells protects against lethal septic shock.
Barker, R. N. et al. Antigen presentation by macrophages is enhanced by the uptake of necrotic, but not apoptotic, cells. Clin. Exp. Immunol. 127, 220–225 (2002).
Mevorach, D., Zhou, J. L., Song, X. & Elkon, K. B. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J. Exp. Med. 188, 387–392 (1998).
Ramos, G. C. et al. Apoptotic mimicry: phosphatidylserine liposomes reduce inflammation through activation of peroxisome proliferator-activated receptors (PPARs) in vivo. Br. J. Pharmacol. 151, 844–850 (2007).
Harel-Adar, T. et al. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc. Natl Acad. Sci. USA 108, 1827–1832 (2011).
Rossi, A. G. et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nature Med. 12, 1056–1064 (2006). The first demonstration that pharmacological induction of apoptosis at sites of inflammation can have anti-inflammatory, pro-resolution effects.
Lucas, C. D. et al. Flavones induce neutrophil apoptosis by down-regulation of Mcl-1 via a proteasomal-dependent pathway. FASEB J. 27, 1084–1094 (2013).
McGrath, E. E. et al. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J. Leukoc. Biol. 90, 855–865 (2011).
Moffatt, O. D., Devitt, A., Bell, E. D., Simmons, D. L. & Gregory, C. D. Macrophage recognition of ICAM-3 on apoptotic leukocytes. J. Immunol. 162, 6800–6810 (1999).
Knies, U. E. et al. Regulation of endothelial monocyte-activating polypeptide II release by apoptosis. Proc. Natl Acad. Sci. USA 95, 12322–12327 (1998).
Blume, K. E. et al. Cleavage of annexin A1 by ADAM10 during secondary necrosis generates a monocytic “find-me” signal. J. Immunol. 188, 135–145 (2012).
Arur, S. et al. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell 4, 587–598 (2003).
Scott, R. S. et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411, 207–211 (2001).
Seitz, H. M., Camenisch, T. D., Lemke, G., Earp, H. S. & Matsushima, G. K. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J. Immunol. 178, 5635–5642 (2007).
He, M. et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 12, 358–364 (2011).
Panaretakis, T. et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 28, 578–590 (2009).
Brown, S. et al. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 418, 200–203 (2002).
Nilsson, A. & Oldenborg, P. A. CD47 promotes both phosphatidylserine-independent and phosphatidylserine-dependent phagocytosis of apoptotic murine thymocytes by non-activated macrophages. Biochem. Biophys. Res. Commun. 387, 58–63 (2009).
Acknowledgements
The authors acknowledge funding from the Australian National Health and Medical Research Council (1013584) for I.K.H.P., Wellcome Trust, UK (WT094415) for C.D.L., the Medical Research Council, UK (G0601481 and MR/K013386/1) for A.G.R. and the US National Institutes of Health (GM107848, GM64709, MH096484, and HD074981) for K.S.R.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Professional phagocytes
-
Professional phagocytes such as macrophages and immature dendritic cells can efficiently detect and engulf pathogens and dying cells.
- Non-professional phagocytes
-
Non-professional phagocytes, such as fibroblasts, epithelial cells and endothelial cells, can engulf a variety of particles, including their dying brethren, but their primary function is not phagocytosis.
- Plasma membrane blebs
-
Globular protrusions seen at the plasma membrane. Membrane blebs are dynamic and can occur during cell migration, cytokinesis and apoptosis.
- Apoptotic bodies
-
Subcellular fragments released from apoptotic cells that are approximately 1–5μm in size. Apoptotic bodies are non-uniform membrane-bound particles that contain portions of cytoplasm and fragmented organelles.
- Focal adhesions
-
Macromolecular complexes that function as structural links between the cell and the extracellular matrix. Components of focal adhesion are also important for regulating intracellular signalling.
- Adherens junctions
-
Intercellular macromolecular complexes that mediate cell–cell adhesion. Cadherin and catenin are key components of adherens junctions.
- Cross-presentation
-
A process that describes the ability of antigen-presenting cells to display a peptide fragment from exogenous antigen, through MHC class I molecules, to CD8+ T cells.
- Organelle fragmentation
-
A process during apoptosis that aids the disassembly of organelles into smaller portions. Organelle fragmentation is driven by caspase-mediated cleavage of certain proteins and actomyosin contraction.
- Apoptotic cell-derived microparticles
-
Another category of subcellular fragments released from apoptotic cells that are approximately 0.1–1μm in size. Apoptotic cell-derived microparticles and apoptotic bodies represent a spectrum of membrane-bound apoptotic vesicles characterized mainly by size and density.
- Germinal centre
-
A lymphoid structure that arises within follicles after immunization with, or exposure to, a T cell-dependent antigen. It is specialized for facilitating the development of high-affinity, long-lived plasma cells and memory B cells.
- Aminophospholipid asymmetry of the plasma membrane
-
The distribution of aminophospholipids (such as phosphatidylserine, phosphatidylethanolamine and phosphatidylcholine) between the outer and inner leaflet of the plasma membrane is often asymmetrical and may differ depending on the cell type, activation status and viability. This asymmetry is actively maintained by ATP-dependent processes and compromised by activation of phospholipid scramblases.
- Endoplasmic reticulum stress
-
(ER stress). A response by the ER that results in the disruption of protein folding and in the accumulation of unfolded proteins in the ER.
- Photodynamic therapy
-
A treatment that uses a combination of a specific wavelength of light and a photosensitizing agent to induce the production of reactive oxygen species and cause lethal damage to the cells.
- Pathogen-associated molecular patterns
-
(PAMPs). Molecular signatures that are found in pathogens but not in mammalian cells. Examples include terminally mannosylated and polymannosylated compounds (which bind the mannose receptor) and various microbial components, such as bacterial lipopolysaccharide, hypomethylated DNA, flagellin and double-stranded RNA (all of which bind Toll-like receptors).
- Damage-associated molecular patterns
-
(DAMPs). As a result of cellular stress, cellular damage and non-physiological cell death, DAMPs are released from the degraded stroma (for example, hyaluronate), from the nucleus (for example, high-mobility group box 1 protein), from the cytosol (for example, ATP, uric acid, S100 calcium-binding proteins and heat-shock proteins) and from mitochondria (formylated peptides and mitochondrial DNA). Such DAMPs are thought to elicit both local and systemic inflammatory responses.
- Chronic obstructive pulmonary disease
-
(COPD). A group of diseases characterized by the pathological limitation of airflow in the airway, including chronic bronchitis and emphysema. COPD is most often caused by tobacco smoking but can also be caused by other airborne irritants, such as coal dust, and occasionally by genetic abnormalities, such as α1-antitrypsin deficiency.
- Pulmonary fibrosis
-
A heterogenous group of disorders characterized by diffuse abnormalities of the pulmonary interstitium, with increased and variable inflammation, and fibrosis. Frequently of unknown aetiology, pulmonary fibrosis can also be related to autoimmune disease and secondary to medications.
- Cystic fibrosis
-
An autosomal recessive genetic condition secondary to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR; a chloride channel). This leads to a multisystem disorder with lung, gastrointestinal, endocrine and fertility complications. Chronic infection of the lungs ensues, leading to significant morbidity and mortality.
- Efferocytosis
-
The phagocytic clearance of apoptotic cells.
- Intima
-
The innermost layer of an artery, which consists of loose connective tissue and is covered by a monolayer of endothelium. Atherosclerotic plaques form within the intima.
- C1q
-
A complement protein and a component of the classical complement pathway. C1q is involved in diverse functions including immune function, autoimmunity and facilitates apoptotic cell clearance.
- Statins
-
A family of inhibitors targeting 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, an enzyme that catalyses the conversion of HMG-CoA to L-mevalonate. These molecules are mainly used as cholesterol-lowering drugs, but they also have immunoregulatory and anti-inflammatory properties. L-mevalonate and its metabolites are implicated in cholesterol synthesis and other intracellular pathways.
- Foam cell
-
A macrophage in the arterial wall that ingests oxidized low-density lipoprotein and assumes a foamy appearance. These cells secrete various substances contributing to plaque growth and inflammation.
Rights and permissions
About this article
Cite this article
Poon, I., Lucas, C., Rossi, A. et al. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 14, 166–180 (2014). https://doi.org/10.1038/nri3607
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3607
This article is cited by
-
Emerging biomarkers and potential therapeutics of the BCL-2 protein family: the apoptotic and anti-apoptotic context
Egyptian Journal of Medical Human Genetics (2024)
-
Gasdermin E dictates inflammatory responses by controlling the mode of neutrophil death
Nature Communications (2024)
-
15-Lipoxygenase promotes resolution of inflammation in lymphedema by controlling Treg cell function through IFN-β
Nature Communications (2024)
-
Increased Intestinal Permeability: An Avenue for the Development of Autoimmune Disease?
Exposure and Health (2024)
-
Apoptotic bodies: bioactive treasure left behind by the dying cells with robust diagnostic and therapeutic application potentials
Journal of Nanobiotechnology (2023)