Key Points
-
Intervention studies or prospective observational epidemiological investigations that use incident cancer as an end point are large, lengthy and costly. Similarly, therapeutic trials based on time to recurrence or mortality can require large numbers of patients and a long follow-up.
-
Therefore, studies with surrogate end points — biomarkers of preclinical carcinogenesis — are attractive because they are potentially smaller, shorter and considerably less costly than their counterparts with cancer end points.
-
Studies based on surrogate end points, however, are inherently less reliable than studies with the 'true' end point (for example, incident cancer, cancer recurrence or mortality). It is important to know when the use of surrogate end points is appropriate and when it is not.
-
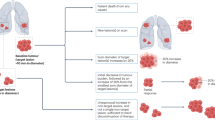
A key issue is whether the test of an association between an exposure (or treatment) and a surrogate end point will reliably indicate whether there is an association between the exposure (treatment) and cancer. Three statistical conditions are needed to establish this: first, the surrogate end point is associated with cancer; second, the exposure (treatment) is associated with the surrogate end point; and third, the surrogate end point 'mediates' the association between exposure (treatment) and cancer. Causal pathway diagrams are useful in understanding these conditions.
-
A second important issue is whether the magnitude of the association between exposure (treatment) and the surrogate end point predicts the magnitude of the association between exposure (treatment) and cancer. A promising approach to this problem relies on the meta-analysis of a series of studies in which exposure (treatment), surrogate end points and cancer are measured concurrently.
-
Even a strong surrogate end point, such as colorectal adenomatous polyps, might not yield definitive results for colorectal cancer.
-
Nevertheless, there are settings, such as preliminary evaluations of potential therapeutic agents or exploratory investigations of aetiological factors, in which data based on surrogate end points could pave the way for subsequent definitive studies.
Abstract
Both experimental and observational studies of cancer need to have an end point. Traditionally, in aetiological and prevention studies, that end point has been the incidence of cancer itself, whereas in therapeutic trials, the end point is usually time to cancer recurrence or death. But cancer takes a long time to develop in an individual and is rare in the population. Therefore, aetiological studies and prevention trials must be large and lengthy to be meaningful. Similarly, many therapeutic trials require a long follow-up of large numbers of patients. Surrogate end points — markers of preclinical cancer or of imminent recurrence — are therefore an attractive alternative. But how can we be sure that a study with a surrogate outcome gives us the right answer about the true end point?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Women's Health Initiative Study Group. Design of the Women's Health Initiative Clinical Trial and Observational Study. Control. Clin. Trials 19, 61–109 (1998).
DeGruttola, V. G. et al. Considerations in the evaluation of surrogate endpoints in clinical trials: summary of a National Institutes of Health workshop. Control. Clin. Trials (in the press).Reviews useful surrogates and Phase III clinical trials, in which their use is often problematic. Sets out statistical concepts and approaches to evaluating surrogate markers, and an agenda for research and resources needed to promote the proper use of surrogates.
New drug, antibiotic and biological drug product regulations: accelerated approval. Proposed Rule. 57 Federal Register 13234–13232, 1992.
Prentice, R. L. Surrogate endpoints in clinical trials: definition and operational criteria. Stat. Med. 8, 431–440 (1989).Defines criteria for the validity of a surrogate marker for testing the hypothesis of no association between a treatment and a true clinical outcome.
Daniels, M. J. & Hughes, M. D. Meta-analysis for the evaluation of potential surrogate markers. Stat. Med. 16, 1965–1982 (1997).
Buyse, M., Molenberghs, G., Burzykowski, T., Renard, D. & Geys, H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics 1, 49–67 (2000).Defines the concept of trial-level validity of a surrogate, based on meta-analysis of a set of trials. Illustrates the use of meta-analytical data for predicting the size of the effect of a new treatment on the true clinical outcome from data on its effect on the surrogate outcome.
Gail, M. H., Pfeiffer, R., Houwelingen, H. C. W. & Carroll, R. J. On meta-analytic assessment of surrogate outcomes. Biostatistics 1, 231–246 (2000).
Temple, R. Are surrogate markers adequate to assess cardiovascular disease drugs? JAMA 282, 790–795 (1999).
Wargovich, M. J. in Prevention and Early Detection of Colorectal Cancer (eds Young, G. P., Rozen, P. & Levin, B) 89–101 (London, W.B. Saunders Co. Ltd, 1996).
Rothman, K. J. & Greenland, S. Modern Epidemiology (Philadelphia, Lippincott–Raven, 1998).
Lipkin, M. et al. Classification and risk assessment of individuals with familial polyposis, Gardner's syndrome, and familial non-polyposis colon cancer from [3H]thymidine labeling patterns in colonic epithelial cells. Cancer Res. 44, 4201–4207 (1984).
Toniolo, P. G. et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J. Natl Cancer Inst. 87, 190–197 (1995).
Hankinson, S. E. et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J. Natl Cancer Inst. 90, 1292–1299 (1998).
Schiffman, M. H. et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J. Natl Cancer Inst. 85, 958–964 (1993).
Schatzkin, A. et al. Lack of effect of a low-fat, high-fiber, diet on the recurrence of colorectal adenomas. N. Engl. J. Med. 342, 1149–1155 (2000).
Holt, P. R. et al. Modulation of abnormal colonic epithelial cell proliferation and differentiation by low-fat dairy foods: a randomized controlled trial. JAMA 280, 1074–1079 (1998).
Prentice, R. et al. Dietary fat reduction and plasma estradiol concentration in healthy premenopausal women. J. Natl Cancer Inst. 82, 129–134 (1990).
Reichman, M. E. et al. Effects of moderate alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J. Natl Cancer Inst. 85, 722–727 (1993).
Baron, J. A. et al. Calcium supplementation and rectal mucosal proliferation: a randomized controlled trial. J. Natl Cancer Inst. 87, 1303–1307 (1995).
Baron, J. A. et al. Calcium supplements for the prevention of colorectal adenomas. N. Engl. J. Med. 340, 101–107 (1999).
Schiffman, M. H. et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J. Natl Cancer Inst. 85, 958–964 (1993).
Freedman, L. S., Graubard, B. I. & Schatzkin, A. Statistical validation of intermediate endpoints for chronic diseases. Stat. Med. 11, 167–178 (1992).
Buyse, M. & Molenberghs, G. Criteria for the validation of surrogate endpoints in randomized experiments. Biometrics 54, 1014–1029 (1998).
DeGruttola, V., Fleming, T., Lin, D. Y. & Coombs, R. Perpsective: validating surrogate markers — are we being naive? J. Infect. Dis. 175, 237–246 (1997).
Schiffman, M. H. & Schatzkin, A. Test reliability is critically important to molecular epidemiology: an example from studies of human papillomavirus infection and cervical neoplasia. Cancer Res. 54, S1944–S1947 (1994). | PubMed |
Franco, E. L. The sexually transmitted disease model for cervical cancer: incoherent epidemiologic findings and the role of misclassification of human papillomavirus infection. Epidemiology 2, 98–106 (1991).
zur Hausen, H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J. Natl Cancer Inst. 92, 690–698 (2000).
Sugarbaker, P. H., Gunderson, L. L. & Wittes, R. E. in Cancer: Principles and Practice of Oncology (eds DeVita, V. T. Jr, Hellman, S. & Rosenberg, S. A.) 795–884 ( J.B. Lippincott Co., Philadelphia, 1985).
Paraskeva, C. et al. Colorectal carcinogenesis: sequential steps in the in vitro immortalization and transformation of human colonic epithelial cells. Anticancer Res. 10, 1189–1200 (1990).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Fleiss, J. L. The Design and Analysis of Clinical Experiments 1–5 (New York, John Wiley & Sons, 1986).
Hankinson, S. E. et al. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer Epidemiol. Biomarker Prev. 4, 649–654 (1995). | PubMed |
Lyles, C. M. et al. Reproducibility and variability of the rectal mucosal proliferation index using proliferating cell nuclear antigen immunohistochemistry. Cancer Epidemiol. Biomarker Prev. 3, 597–605 (1994). | PubMed |
Fleming, T. R. & DeMets, D. L. Surrogate end points in clinical trials: are we being misled? Ann. Intern. Med. 125, 605–613 (1996).Presents several examples from clinical studies of surrogate end point findings that did not agree with true end-point results.
Mitchell, M. F., Hittelman, W. N., Hong, W. K., Lotan, R. & Schottenfeld, D. The natural history of cervical intraepithelial neoplasia: an argument for intermediate endpoint biomarkers. Cancer Epidemiol. Biomarker Prev. 3, 619–626 (1994). | PubMed |
Bostwick, D. G. Prostatic intraepithelial neoplasia is a risk factor for cancer. Semin. Urol. Oncol. 17, 187–198 (1999).
Mutter, G. L. Endometrial intraepithelial neoplasia (EIN): will it bring order to chaos? Gynecol. Oncol. 76, 287–290 (2000).
Schatzkin, A., Freedman, L. S., Dawsey, S. M. & Lanza, E. Interpreting precursor studies: what polyp trials tell us about large bowel cancer. J. Natl Cancer Inst. 86, 1053–1057 (1994).
Misset, J. L. et al. Regression of bronchial epidermoid metaplasia in heavy smokers with etretinate treatment. Cancer Detect. Prev. 9, 167–170 (1986).
Dawsey, S. M. et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer 83, 220–231 (1998).
Saftlas, A. F. et al. Mammographic parenchymal patterns as indicators of breast cancer risk. Am. J. Epidemiol. 129, 518–526 (1989).
Karlan, B. Y. Screening for ovarian cancer: what are the optimal surrogate endpoints for clinical trials? J. Cell Biochem. 23 (Suppl.), 227–232 (1995). | PubMed |
Baron, J. A. et al. Epidemiological use of rectal proliferation measures. Cancer Epidemiol. Biomarker Prev. 4, 57–61 (1995).
Bedi, A. et al. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 55, 1811–1816 (1995).
Tsiatis, A. A., DeGruttola, V. & Wulfsohn, M. S. Modeling the relationship of survival to longitudinal data measured with error. Applications to survival and CD4 counts in patients with AIDS. J. Am. Stat. Assoc. 90, 27–37 (1995).
Ruiz, L. et al. Plasma HIV-1 RNA as a predictor of the efficacy of adding zalcitabine to a previous regimen with zidovudine. Antivir. Ther. 1, 220–224 (1996).
Fearon, E. R. Genetic alterations underlying colorectal tumorigenesis. Cancer Surveys 12, 119–136 (1992).
Counts, J. L. & Goodman, J. I. Alterations in DNA methylation may play a variety of roles in carcinogenesis. Cell 83, 13–15 (1995).
Brown, P. O. & Botstein, D. Exploring the new world of the genome with DNA microarrays. Nature Genet. 21, S33–S37 (1999). | PubMed |
Groopman, J. D., Wogan, G. N., Roebuck, B. D. & Kensler, T. W. Molecular biomarkers for aflatoxins and their application to human cancer prevention. Cancer Res. 54, S1907–S1911 (1994). | PubMed |
Schiffman, M. H. Recent progress in defining the epidemiology of human papillomavirus infection and cervical neoplasia. J. Natl Cancer Inst. 84, 394–398 (1992).
Munoz, N. Is Helicobacter pylori a cause of gastric cancer? An appraisal of the seroepidemiological evidence. Cancer Epidemiol. Biomarker Prev. 3, 445–451 (1994). | PubMed |
Blattner, W. A. in Viral Infections in Humans 3rd edn (ed. Evans, A. S) 545–592 (Plenum Medical Book Co., New York, 1989).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Dorgan, J. F. et al. Relations of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol. Biomarker Prev. 5, 533–539 (1996). | PubMed |
Author information
Authors and Affiliations
Related links
Related links
DATABASES
FURTHER INFORMATION
FDA Center for Drug Evaluation and Research — approval of drugs based on surrogate end points
NCI Division of Cancer Epidemiology and Genetics
NCI Early Detection Research Network
Tutorials on randomized clinical studies from Beth Israel Deaconess Biometrics Center
Glossary
- INTERVENTION STUDIES
-
Also known as clinical trials, these are clinical experiments in which the types of treatment and their allocation to study participants are under the control of the investigator. Usually the treatments are randomly allocated to study participants.
- PROSPECTIVE OBSERVATIONAL STUDIES
-
Studies of well-defined groups (cohorts) of individuals for whom exposure data are available initially and for whom follow-up procedures are in place to determine if and when subsequent disease end points arise. The exposures and their allocations to cohort members are not controlled by the investigator.
- PROLIFERATION INDICES
-
Measures of the rate of cell turnover or DNA synthesis derived from one of several proliferation bioassays that are currently available.
- RELATIVE RISK
-
An epidemiological measure of treatment effect in an intervention study (clinical trial) or exposure association in a non-experimental observational study. The relative risk is the ratio of risk in an exposed (treated) group to the risk in an unexposed (control) group.
- ATTRIBUTABLE PROPORTION
-
(AP). An epidemiological measure of the proportion of all disease cases that is attributable to exposure. The attributable proportion is 1.0 minus the ratio of risk in an unexposed population to the risk in the mixed population of exposed and unexposed individuals. In the context of surrogate markers of cancer, the AP can indicate the proportion of incident cancer that is attributable to marker positivity.
- MULTIPLE REGRESSION
-
A statistical regression model with more than one independent variable.
- STATISTICAL REGRESSION MODEL
-
A statistical approach to quantifying the relationship between an end point ('dependent variable') and other factors ('independent variables') such as treatments or exposures. Regression models are available for continuous, dichotomous, and survival end points.
Rights and permissions
About this article
Cite this article
Schatzkin, A., Gail, M. The promise and peril of surrogate end points in cancer research. Nat Rev Cancer 2, 19–27 (2002). https://doi.org/10.1038/nrc702
Issue Date:
DOI: https://doi.org/10.1038/nrc702
This article is cited by
-
Meat-derived carcinogens, genetic susceptibility and colorectal adenoma risk
Genes & Nutrition (2014)
-
Mammographic density and breast cancer risk: current understanding and future prospects
Breast Cancer Research (2011)
-
Circulating Sex Hormones and Mammographic Breast Density among Postmenopausal Women
Hormones and Cancer (2011)