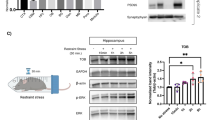
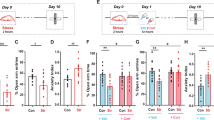
Abstract
Many of the behavioral consequences of stress are mediated by the activation of the glucocorticoid receptor by stress-induced high levels of glucocorticoid hormones. To explore the molecular mechanisms of these effects, we combined in vivo and in vitro approaches. We analyzed mice carrying a brain-specific mutation (GRNesCre) in the glucocorticoid receptor gene (GR, also called Nr3c1) and cell lines that either express endogenous glucocorticoid receptor or carry a constitutively active form of the receptor (ΔGR) that can be transiently induced. In the hippocampus of the mutant mice after stress, as well as in the cell lines, activation of glucocorticoid receptors greatly increased the expression and enzymatic activity of proteins in the MAPK signaling pathway and led to an increase in the levels of both Egr-1 mRNA and protein. In parallel, inhibition of the MAPK pathway within the hippocampus abolished the increase in contextual fear conditioning induced by glucocorticoids. The present results provide a molecular mechanism for the stress-related effects of glucocorticoids on fear memories.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
De Kloet, E.R. Hormones, brain and stress. Endocr. Regul. 37, 51–68 (2003).
McEwen, B.S., De Kloet, E.R. & Rostene, W. Adrenal steroid receptors and actions in the nervous system. Physiol. Rev. 66, 1121–1188 (1986).
McEwen, B.S. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 886, 172–189 (2000).
Piazza, P.V. & Le Moal, M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res. Brain Res. Rev. 25, 359–372 (1997).
Sapolsky, R.M. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry 57, 925–935 (2000).
De Kloet, E.R., Oitzl, M.S. & Joels, M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 22, 422–426 (1999).
McGaugh, J.L. & Roozendaal, B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol. 12, 205–210 (2002).
Holsboer, F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23, 477–501 (2000).
Wei, Q. et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc. Natl. Acad. Sci. USA 101, 11851–11856 (2004).
Funder, J.W. Glucocorticoid receptors. J. Steroid Biochem. Mol. Biol. 43, 389–394 (1992).
Miner, J.N. & Yamamoto, K.R. Regulatory crosstalk at composite response elements. Trends Biochem. Sci. 16, 423–426 (1991).
Tronche, F., Kellendonk, C., Reichardt, H.M. & Schutz, G. Genetic dissection of glucocorticoid receptor function in mice. Curr. Opin. Genet. Dev. 8, 532–538 (1998).
Chang, L. & Karin, M. Mammalian MAP kinase signalling cascades. Nature 410, 37–40 (2001).
Marais, R. & Marshall, C.J. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 27, 101–125 (1996).
O'Donovan, K.J., Tourtellotte, W.G., Millbrandt, J. & Baraban, J.M. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 22, 167–173 (1999).
Meller, E. et al. Region-specific effects of acute and repeated restraint stress on the phosphorylation of mitogen-activated protein kinases. Brain Res. 979, 57–64 (2003).
Sananbenesi, F., Fischer, A., Schrick, C., Spiess, J. & Radulovic, J. Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J. Neurosci. 23, 11436–11443 (2003).
Guzowski, J.F., Setlow, B., Wagner, E.K. & McGaugh, J.L. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J. Neurosci. 21, 5089–5098 (2001).
Hall, J., Thomas, K.L. & Everitt, B.J. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J. Neurosci. 21, 2186–2193 (2001).
Jouvert, P., Dietrich, J.B., Aunis, D. & Zwiller, J. Differential rat brain expression of EGR proteins and of the transcriptional corepressor NAB in response to acute or chronic cocaine administration. Neuromolecular Med. 1, 137–151 (2002).
Moratalla, R., Robertson, H.A. & Graybiel, A.M. Dynamic regulation of NGFI-A (zif268, egr1) gene expression in the striatum. J. Neurosci. 12, 2609–2622 (1992).
Valjent, E., Pages, C., Herve, D., Girault, J.A. & Caboche, J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur. J. Neurosci. 19, 1826–1836 (2004).
Atkins, C.M., Selcher, J.C., Petraitis, J.J., Trzaskos, J.M. & Sweatt, J.D. The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1, 602–609 (1998).
Blum, S., Moore, A.N., Adams, F. & Dash, P.K. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J. Neurosci. 19, 3535–3544 (1999).
Bozon, B., Davis, S. & Laroche, S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron 40, 695–701 (2003).
Jones, M.W. et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat. Neurosci. 4, 289–296 (2001).
Kelly, A., Laroche, S. & Davis, S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 23, 5354–5360 (2003).
Tronche, F. et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23, 99–103 (1999).
Storm, S.M., Cleveland, J.L. & Rapp, U.R. Expression of raf family proto-oncogenes in normal mouse tissues. Oncogene 5, 345–351 (1990).
Kitchener, P., Di Blasi, F., Borrelli, E. & Piazza, P.V. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur. J. Neurosci. 19, 1837–1846 (2004).
Gossen, M. et al. Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769 (1995).
Yuryev, A. & Wennogle, L.P. The RAF family: an expanding network of post-translational controls and protein-protein interactions. Cell Res. 8, 81–98 (1998).
Favata, M.F. et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623–18632 (1998).
Strawhecker, J.M., Betz, N.A., Neades, R.Y., Houser, W. & Pelling, J.C. Binding of the 97 kDa glucocorticoid receptor to the 5′ upstream flanking region of the mouse c-Ha-ras oncogene. Oncogene 4, 1317–1322 (1989).
Lee, J.E., Beck, T.W., Wojnowski, L. & Rapp, U.R. Regulation of A-raf expression. Oncogene 12, 1669–1677 (1996).
Beato, M., Herrlich, P. & Schutz, G. Steroid hormone receptors: many actors in search of a plot. Cell 83, 851–857 (1995).
Cella, N., Groner, B. & Hynes, N.E. Characterization of Stat5a and Stat5b homodimers and heterodimers and their association with the glucocortiocoid receptor in mammary cells. Mol. Cell. Biol. 18, 1783–1792 (1998).
Widen, C., Zilliacus, J., Gustafsson, J.A. & Wikstrom, A.C. Glucocorticoid receptor interaction with 14–3-3 and Raf-1, a proposed mechanism for cross-talk of two signal transduction pathways. J. Biol. Chem. 275, 39296–39301 (2000).
Qiu, J. et al. Rapid activation of ERK1/2 mitogen-activated protein kinase by corticosterone in PC12 cells. Biochem. Biophys. Res. Commun. 287, 1017–1024 (2001).
Richter-Levin, G. & Akirav, I. Amygdala-hippocampus dynamic interaction in relation to memory. Mol. Neurobiol. 22, 11–20 (2000).
Oitzl, M.S., Reichardt, H.M., Joels, M. & De Kloet, E.R. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc. Natl. Acad. Sci. USA 98, 12790–12795 (2001).
Impey, S., Obrietan, K. & Storm, D.R. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron 23, 11–14 (1999).
Kelleher, R.J., III, Govindarajan, A., Jung, H.Y., Kang, H. & Tonegawa, S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116, 467–479 (2004).
Lee, J.L., Everitt, B.J. & Thomas, K.L. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304, 839–843 (2004).
Marinelli, M. & Piazza, P.V. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur. J. Neurosci. 16, 387–394 (2002).
Deroche-Gamonet, V. et al. The glucocorticoid receptor as a potential target to reduce cocaine abuse. J. Neurosci. 23, 4785–4790 (2003).
Piazza, P.V. & Le Moal, M. The role of stress in drug self-administration. Trends Pharmacol. Sci. 19, 67–74 (1998).
Lu, L., Dempsey, J., Liu, S.Y., Bossert, J.M. & Shaham, Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J. Neurosci. 24, 1604–1611 (2004).
Godowski, P.J., Rusconi, S., Miesfeld, R. & Yamamoto, K.R. Glucocorticoid receptor mutants that are constitutive activators of transcriptional enhancement. Nature 325, 365–368 (1987).
Desmedt, A., Garcia, R. & Jaffard, R. Differential modulation of changes in hippocampal-septal synaptic excitability by the amygdala as a function of either elemental or contextual fear conditioning in mice. J. Neurosci. 18, 480–487 (1998).
Acknowledgements
We are grateful to Y. Shaham for comments on the manuscript and to M. Petit and A. Le Roux for technical help. This work was supported by INSERM, Bordeaux Institute for Neurosciences (IFR8), the Université Victor Segalen–Bordeaux 2 and Conseil Régional d'Aquitaine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Schematic representation of the proposed molecular mechanism by which stress-activated GRs mediate some of the behavioral effects of stress. Following stress, activation of the GR by glucocorticoids rapidly increases (within 30 min) the levels of Egr-1 via a MAPK-independent pathway potentially acting on the Egr-1 promoter either directly or through the modulation of other transcription factors (grey circle). In parallel, stress-activated GR also rapidly increases the expression of proteins up-stream in MAPK pathway (Ras and Raf-1). This effect is followed by a delayed activation of Erk1/2 (maximal at 2 hours), which in turn results in sustained increased expression of Egr-1, even when the glucocorticoid signal has been turned off. This increase in Egr-1 expression can mediate some of the behavioral effects of stress, such as the enhanced memory of emotionally charged experience. (PDF 1306 kb)
Rights and permissions
About this article
Cite this article
Revest, JM., Di Blasi, F., Kitchener, P. et al. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci 8, 664–672 (2005). https://doi.org/10.1038/nn1441
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1441
This article is cited by
-
Berberine ameliorates depression-like behaviors in mice via inhibiting NLRP3 inflammasome-mediated neuroinflammation and preventing neuroplasticity disruption
Journal of Neuroinflammation (2023)
-
Stress-related cellular pathophysiology as a crosstalk risk factor for neurocognitive and psychiatric disorders
BMC Neuroscience (2023)
-
Neurocognitive effects of stress: a metaparadigm perspective
Molecular Psychiatry (2023)
-
PAI-1 protein is a key molecular effector in the transition from normal to PTSD-like fear memory
Molecular Psychiatry (2021)
-
Differential and unique patterns of synaptic miRNA expression in dorsolateral prefrontal cortex of depressed subjects
Neuropsychopharmacology (2021)