Abstract
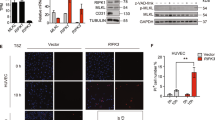
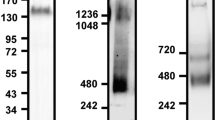
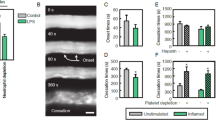
Inflammatory recruitment of leukocytes is governed by dynamic interactions between integrins and endothelial immunoglobulin superfamily (IgSF) proteins. We have identified the IgSF member junctional adhesion molecule 1 (JAM-1) as a ligand of the β2 integrin lymphocyte function–associated antigen 1 (LFA-1). Under static and physiological flow conditions, JAM-1 contributed to LFA-1–dependent transendothelial migration of T cells and neutrophils as well as LFA-1–mediated arrest of T cells. The latter was triggered by chemokines on endothelium that was stimulated with cytokines to redistribute JAM-1 from the tight junctions. Transfectants expressing JAM-1 supported LFA-1–mediated adhesion of leukocytes, which required the membrane-proximal Ig-like domain 2 of JAM-1. Thus, JAM-1 is a counter-receptor for LFA-1 that is ideally situated to guide and control transmigration during leukocyte recruitment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Springer, T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314 (1994).
Weber, C., Kitayama, J. & Springer, T. A. Differential regulation of β1 and β2 integrin avidity by chemoattractants in eosinophils. Proc. Natl Acad. Sci. USA 93, 10939–10944 (1996).
Campbell, J. J. et al. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 279, 381–384 (1998).
Smith, C. W., Marlin, S. D., Rothlein, R., Toman, C. & Anderson, D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J. Clin. Invest. 83, 2008–2017 (1989).
Weber, C., Lu, C. F., Casasnovas, J. M. & Springer, T. A. Role of αLβ2 integrin avidity in transendothelial chemotaxis of mononuclear cells. J. Immunol. 159, 3968–3975 (1997).
Rothlein, R., Dustin, M. L., Marlin, S. D. & Springer, T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J. Immunol. 137, 1270–1274 (1986).
Marlin, S. D. & Springer, T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 51, 813–819 (1987).
Nortamo, P., Salcedo, R., Timonen, T., Patarroyo, M. & Gahmberg, C. G. A monoclonal antibody to the human leukocyte adhesion molecule intercellular adhesion molecule-2. Cellular distribution and molecular characterization of the antigen. J. Immunol. 146, 2530–2535 (1991).
de Fougerolles, A. R. & Springer, T. A. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J. Exp. Med. 175, 185–190 (1992).
Bleijs, D. A., Binnerts, M. E., van Vliet, S. J., Figdor, C. G. & van Kooyk, Y. Low-affinity LFA-1/ICAM-3 interactions augment LFA-1/ICAM-1-mediated T cell adhesion and signaling by redistribution of LFA-1. J. Cell Sci. 113, 391–400 (2000).
Diamond, M. S., Staunton, D. E., Marlin, S. D. & Springer, T. A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell 65, 961–971 (1991).
Elices, M. J. et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell 60, 577–584 (1990).
Berlin, C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Piali, L. et al. CD31/PECAM-1 is a ligand for αvβ3 integrin involved in adhesion of leukocytes to endothelium. J. Cell Biol. 130, 451–460 (1995).
Martin-Padura, I. et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 142, 117–127 (1998).
Ozaki, H. et al. Combined treatment of TNF-α and IFN-γ causes redistribution of junctional adhesion molecule in human endothelial cells. J. Immunol. 163, 553–557 (1999).
Williams, L. A., Martin-Padura, I., Dejana, E., Hogg, N. & Simmons, D. L. Identification and characterisation of human junctional adhesion molecule (JAM). Mol. Immunol. 36, 1175–1188 (1999).
Bazzoni, G. et al. Homophilic interaction of junctional adhesion molecule. J. Biol. Chem. 275, 30970–30976 (2000).
Ebnet, K., Schulz, C. U., Meyer, Z. B. M., Pendl, G. G. & Vestweber, D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J. Biol. Chem. 275, 27979–27988 (2000).
Liang, T. W. et al. Characterization of huJAM: evidence for involvement in cell-cell contact and tight junction regulation. Am. J. Physiol. Cell Physiol. 279, 1733–1743 (2000).
Malergue, F. et al. A novel immunoglobulin superfamily junctional molecule expressed by antigen presenting cells, endothelial cells and platelets. Mol. Immunol. 35, 1111–1119 (1998).
Sobocka, M. B. et al. Cloning of the human platelet F11 receptor: a cell adhesion molecule member of the immunoglobulin superfamily involved in platelet aggregation. Blood 95, 2600–2609 (2000).
Del Maschio, A. et al. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM). J. Exp. Med. 190, 1351–1356 (1999).
Muller, W. A., Weigl, S. A., Deng, X. & Phillips, D. M. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 178, 449–460 (1993).
Weber, K. S., York, M. R., Springer, T. A. & Klickstein, L. B. Characterization of lymphocyte function-associated antigen 1 (LFA-1)-deficient T cell lines: the αLβ2 subunits are interdependent for cell surface expression. J. Immunol. 158, 273–279 (1997).
Weber, K. S., Klickstein, L. B. & Weber, C. Specific activation of leukocyte β2 integrins lymphocyte function-associated antigen-1 and Mac-1 by chemokines mediated by distinct pathways via the α subunit cytoplasmic domains. Mol. Biol. Cell 10, 861–873 (1999).
Nanki, T. et al. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J. Immunol. 165, 6590–6598 (2000).
Luscinskas, F. W., Ding,H. & Lichtman, A. H. P-selectin and vascular cell adhesion molecule 1 mediate rolling and arrest, respectively, of CD4+ T lymphocytes on tumor necrosis factor α-activated vascular endothelium under flow. J. Exp. Med. 181, 1179–1186 (1995).
Peled, A. et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J. Clin. Invest. 104, 1199–1211 (1999).
Gopalan, P. K. et al. Preferential sites for stationary adhesion of neutrophils to cytokine-stimulated HUVEC under flow conditions. J. Leukoc. Biol. 68, 47–57 (2000).
Kornecki, E., Walkowiak, B., Naik, U. P. & Ehrlich, Y. H. Activation of human platelets by a stimulatory monoclonal antibody. J. Biol. Chem. 265, 10042–10048 (1990).
Naik, U. P., Ehrlich, Y. H. & Kornecki, E. Mechanisms of platelet activation by a stimulatory antibody: cross-linking of a novel platelet receptor for monoclonal antibody F11 with the FcγRII receptor. Biochem. J. 310, 155–162 (1995).
Cunningham, S. A. et al. A novel protein with homology to the junctional adhesion molecule. Characterization of leukocyte interactions. J. Biol. Chem. 275, 34750–34756 (2000).
Palmeri, D., van Zante, A., Huang, C. C., Hemmerich, S. & Rosen, S. D. Vascular endothelial junction-associated molecule, a novel member of the immunoglobulin superfamily, is localized to intercellular boundaries of endothelial cells. J. Biol. Chem. 275, 19139–19145 (2000).
Aurrand-Lions, M.A., Duncan, L., Du, P.L. & Imhof, B.A. Cloning of JAM-2 and JAM-3: an emerging junctional adhesion molecular family? Curr. Top. Microbiol. Immunol. 251, 91–98 (2000).
Aurrand-Lions, M. A., Duncan, L., Ballestrem, C. & Imhof, B. A. JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J. Biol. Chem. 276, 2733–2741 (2001).
Bazzoni, G. et al. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J. Biol. Chem. 275, 20520–20526 (2000).
Ozaki, H. et al. Junctional adhesion molecule (JAM) is phosphorylated by protein kinase C upon platelet activation. Biochem. Biophys. Res. Commun. 276, 873–878 (2000).
Naik, U. P., Naik, M. U., Eckfeld, K., Martin-DeLeon, P. & Spychala, J. Characterization and chromosomal localization of JAM-1, a platelet receptor for a stimulatory monoclonal antibody. J. Cell Sci. 114, 539–547 (2001).
Ayalon, O., Sabanai, H., Lampugnani, M. G., Dejana, E. & Geiger, B. Spatial and temporal relationships between cadherins and PECAM-1 in cell-cell junctions of human endothelial cells. J. Cell Biol. 126, 247–258 (1994).
Kostrewa, D. et al. X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. EMBO J. 20, 4391–4398 (2001).
Klickstein, L. B., York, M. R., Fougerolles, A. R. & Springer, T. A. Localization of the binding site on intercellular adhesion molecule-3 (ICAM-3) for lymphocyte function-associated antigen 1 (LFA–1). J. Biol. Chem. 271, 23920–23927 (1996).
Horton, R. M., Hunt, H. D., Ho, S. N., Pullen, J. K. & Pease, L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77, 61–68 (1989).
Weber, K. S., von Hundelshausen, P., Clark-Lewis, I., Weber, P. C. & Weber, C. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur. J. Immunol. 29, 700–712 (1999).
Weber, C. et al. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and TH1-like/CD45RO+ T cells. Blood 97, 1144–1146 (2001).
Davis, H. L., Michel, M. L. & Whalen, R. G. Use of plasmid DNA for direct gene transfer and immunization. Ann. NY Acad. Sci. 772, 21–29 (1995).
Weber, C., Alon, R., Moser, B. & Springer, T. A. Sequential regulation of α4β1 and α5β1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J. Cell Biol. 134, 1063–1073 (1996).
Kukreti, S., Konstantopoulos, K., Smith, C. W. & McIntire, L. V. Molecular mechanisms of monocyte adhesion to interleukin-1β-stimulated endothelial cells under physiologic flow conditions. Blood 89, 4104–4111 (1997).
Acknowledgements
We thank P. C. Weber for continuous support, G. Heiss for initial support and advice and N. Gellert for expert technical assistance. Supported by Deutsche Forschungsgemeinschaft (grant WE-1913/2 to C. W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ostermann, G., Weber, K., Zernecke, A. et al. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol 3, 151–158 (2002). https://doi.org/10.1038/ni755
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni755
This article is cited by
-
Investigating the potential anti-depressive mechanisms of statins: a transcriptomic and Mendelian randomization analysis
Translational Psychiatry (2023)
-
JAM-A Overexpression in Human Umbilical Cord-Derived Mesenchymal Stem Cells Accelerated the Angiogenesis of Diabetic Wound By Enhancing Both Paracrine Function and Survival of Mesenchymal Stem Cells
Stem Cell Reviews and Reports (2023)
-
Systematic analysis of prognostic significance, functional enrichment and immune implication of STK10 in acute myeloid leukemia
BMC Medical Genomics (2022)
-
Effect of anemoside B4 on milk whey in clinical mastitis-affected cows elucidated using tandem mass tag (TMT)-based quantitative proteomics
Scientific Reports (2022)
-
STING agonism enhances anti-tumor immune responses and therapeutic efficacy of PARP inhibition in BRCA-associated breast cancer
npj Breast Cancer (2022)