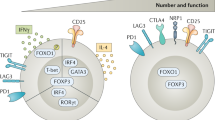
Abstract
It is now well established that regulatory T (TR) cells can inhibit harmful immunopathological responses directed against self or foreign antigens. However, many key aspects of TR cell biology remain unresolved, especially with regard to their antigen specificities and the cellular and molecular pathways involved in their development and mechanisms of action. We will review here recent findings in these areas, outline a model for how TR cells may inhibit the development of immune pathology and discuss potential therapeutic benefits that may arise from the manipulation of TR cell function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gershon, R. K. A disquisition on suppressor T cells. Transplant. Rev. 26, 170–185 (1975).
Mason, D. & Powrie, F. Control of immune pathology by regulatory T cells. Curr. Opin. Immunol. 10, 649–655 (1998).
Shevach, E. M. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 18, 423–449 (2000).
Sakaguchi, S. Animal models of autoimmunity and their relevance to human diseases. Curr. Opin. Immunol. 12, 684–690 (2000).
Roncarolo, M. G. & Levings, M. K. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr. Opin. Immunol. 12, 676–683 (2000).
Fowell, D. & Mason, D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J. Exp. Med. 177, 627–636 (1993).
Hafler, D. A. & Weiner, H. L. Immunologic mechanisms and therapy in multiple sclerosis. Immunol. Rev. 144, 75–107 (1995).
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995).
Hemmer, B., Vergelli, M., Pinilla, C., Houghten, R. & Martin, R. Probing degeneracy in T-cell recognition using peptide combinatorial libraries. Immunol. Today. 19, 163–168 (1998).
Mason, D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today 19, 395–404 (1998).
Hausmann, S. & Wucherpfennig, K. W. Activation of autoreactive T cells by peptides from human pathogens. Curr. Opin. Immunol. 9, 831–838 (1997).
Schwartz, R. H. Models of T cell anergy: is there a common molecular mechanism? J. Exp. Med. 184, 1–8 (1996).
Miller, J. F. & Basten, A. Mechanisms of tolerance to self. Curr. Opin. Immunol. 8, 815–821 (1996).
Zinkernagel, R. M. et al. Antigen localisation regulates immune responses in a dose- and time- dependent fashion: a geographical view of immune reactivity. Immunol. Rev. 156, 199–209 (1997).
Coutinho, A., Salaun, J., Corbel, C., Bandeira, A. & Le Douarin, N. The role of thymic epithelium in the establishment of transplantation tolerance. Immunol. Rev. 133, 225–240 (1993).
Le Douarin, N. et al. Evidence for a thymus-dependent form of tolerance that is not based on elimination or anergy of reactive T cells. Immunol. Rev. 149, 35–53 (1996).
Asano, M., Toda, M., Sakaguchi, N. & Sakaguchi, S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184, 387–396 (1996).
Seddon, B. & Mason, D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor β and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4+CD45RC− cells and CD4+CD8− thymocytes. J. Exp. Med. 189, 279–288 (1999).
Olivares-Villagomez, D., Wang, Y. & Lafaille, J. J. Regulatory CD4+ T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 188, 1883–1894 (1998).
Van de Keere, F. & Tonegawa, S. CD4+ T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice. J. Exp. Med. 188, 1875–1882 (1998).
Mordes, J. P. et al. Transfusions enriched for W3/25+ helper/inducer T lymphocytes prevent spontaneous diabetes in the BB/W rat. Diabetologia 30, 22–26 (1987).
Powrie, F., Leach, M. W., Mauze, S., Caddle, L. B. & Coffman, R. L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5, 1461–1471 (1993).
Read, S., Malmstrom, V. & Powrie, F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192, 295–302 (2000).
Stockinger, G., Barthlott, T. & Kassiotis, G. T cell regulation: a special job or everyone's responsibility? Nature Immunol. 2, 757–758 (2001).
Takahashi, T. et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10, 1969–1980 (1998).
Thornton, A. E. & Shevach, E. M. CD4+ CD25+ Immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188, 287–296 (1998).
Suri-Payer, E., Amar, Z. A., Thornton, A. M. & Shevach, E. M. CD4+ CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160, 1212–1218 (1998).
Salomon, B. et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12, 431–440 (2000).
Takahashi, T. et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192, 303–310 (2000).
Papiernik, M., de Moraes, M. L., Pontoux, C., Vasseur, F. & Penit, C. Regulatory CD4 T cells: expression of IL-2R α chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 10, 371–378 (1998).
Stephens, L. A. & Mason, D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J. Immunol. 165, 3105–3110 (2000).
Hara, M. et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 166, 3789–3796 (2001).
Gao, Q., Rouse, T. M., Kazmerzak, K. & Field, E. H. CD4+CD25+ cells regulate CD8 cell anergy in neonatal tolerant mice. Transplantation 68, 1891–1897 (1999).
Taylor, P. A., Noelle, R. J. & Blazar, B. R. CD4+CD25+ Immune Regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 193, 1311–1318 (2001).
Shimizu, J., Yamazaki, S. & Sakaguchi, S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163, 5211–5218 (1999).
Annacker, O. et al. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 166, 3008–3018 (2001).
Itoh, M. et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162, 5317–5326 (1999).
Stephens, L. A., Mottet, C., Mason, D. & Powrie, F. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 31, 1247–1254 (2001).
Taams, L. S. et al. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 31, 1122–1131 (2001).
Dieckmann, D., Plottner, H., Berchtold, S., Berger, T. & Schuler, G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193, 1303–1310 (2001).
Levings, M. K., Sangregorio, R. & Roncarolo, M. G. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193, 1295–1302 (2001).
Jonuleit, H. et al. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193, 1285–1294 (2001).
Olivares-Villagomez, D., Wensky, A. K., Wang, Y. & Lafaille, J. J. Repertoire requirements of CD4+ T cells that prevent spontaneous autoimmune encephalomyelitis. J. Immunol. 164, 5499–5507 (2000).
Jenkins, M. K. & Schwartz, R. H. Antigen presentation by chemically modified splenocytes induces antigen- specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 165, 302–319 (1987).
Chai, J. G. et al. Anergic T cells act as suppressor cells in vitro and in vivo. Eur. J. Immunol. 29, 686–692 (1999).
Taams, L. S. et al. Anergic T cells actively suppress T cell responses via the antigen- presenting cell. Eur. J. Immunol. 28, 2902–2912 (1998).
Vendetti, S. et al. Anergic T cells inhibit the antigen-presenting function of dendritic cells. J. Immunol. 165, 1175–1181 (2000).
Buer, J. et al. Interleukin 10 secretion and impaired effector function of major histocompatibility complex class II-restricted T cells anergized in vivo. J. Exp. Med. 187, 177–183 (1998).
Sundstedt, A. et al. Immunoregulatory role of IL-10 during superantigen-induced hyporesponsiveness in vivo. J. Immunol. 158, 180–186 (1997).
Groux, H., Bigler, M., de Vries, J. E. & Roncarolo, M. G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 184, 19–29 (1996).
Groux, H. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 (1997).
Cottrez, F., Hurst, S. D., Coffman, R. L. & Groux, H. T regulatory cells 1 inhibit a Th2-specific response in vivo. J. Immunol. 165, 4848–4853 (2000).
Chen, Y., Kuchroo, V. K., Inobe, J., Hafler, D. A. & Weiner, H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 265, 1237–1240 (1994).
Neurath, M. F. et al. Experimental granulomatous colitis in mice is abrogated by induction of TGF-β-mediated oral tolerance. J. Exp. Med. 183, 2605–2616 (1996).
Weiner, H. L. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol. Today 18, 335–343 (1997).
Waldmann, H. & Cobbold, S. Regulating the immune response to transplants. A role for CD4+ regulatory cells? Immunity 14, 399–406 (2001).
Zhai, Y. & Kupiec-Weglinski, J. W. What is the role of regulatory T cells in transplantation tolerance? Curr. Opin. Immunol. 11, 497–503 (1999).
Cobbold, S. & Waldmann, H. Infectious tolerance. Curr. Opin. Immunol. 10, 518–524 (1998).
Davies, J. D. et al. CD4+ CD45RB low-density cells from untreated mice prevent acute allograft rejection. J. Immunol. 163, 5353–5357 (1999).
Seddon, B. & Mason, D. The third function of the thymus. Immunol. Today 21, 95–99 (2000).
Saoudi, A., Seddon, B., Fowell, D. & Mason, D. The thymus contains a high frequency of cells that prevent autoimmune diabetes on transfer into prediabetic recipients. J. Exp. Med. 184, 2393–2398 (1996).
Herbelin, A., Gombert, J. M., Lepault, F., Bach, J. F. & Chatenoud, L. Mature mainstream TCRαβ+CD4+ thymocytes expressing L-selectin mediate “active tolerance” in the nonobese diabetic mouse. J. Immunol. 161, 2620–2628 (1998).
Singh, B. et al. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182, (in the press, 2001).
Modigliani, Y., Bandeira, A. & Coutinho, A. A model for developmentally acquired thymus-dependent tolerance to central and peripheral antigens. Immunol. Rev. 149, 155–120 (1996).
Jordan, M. S., Riley, M. P., von Boehmer, H. & Caton, A. J. Anergy and suppression regulate CD4+ T cell responses to a self peptide. Eur. J. Immunol. 30, 136–144 (2000).
Jordan, M. S. et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature Immunol. 2, 301–306 (2001).
Suri-Payer, E. et al. Post-thymectomy autoimmune gastritis: fine specificity and pathogenicity of anti-H/K ATPase-reactive T cells. Eur. J. Immunol. 29, 669–677 (1999).
Kumanogoh, A. et al. Increased T cell autoreactivity in the absence of CD40-CD40 ligand interactions: a role of CD40 in regulatory T cell development. J. Immunol. 166, 353–360 (2001).
Kuniyasu, Y. et al. Naturally anergic and suppressive CD25+CD4+ T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int. Immunol. 12, 1145–1155 (2000).
Seddon, B. & Mason, D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J. Exp. Med. 189, 877–882 (1999).
Duchmann, R. et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin. Exp. Immunol. 102, 448–455 (1995).
Khoo, U. Y., Proctor, I. E. & Macpherson, A. J. CD4+ T cell down-regulation in human intestinal mucosa: evidence for intestinal tolerance to luminal bacterial antigens. J. Immunol. 158, 3626–3634 (1997).
Taguchi, O. et al. Tissue-specific suppressor T cells involved in self-tolerance are activated extrathymically by self-antigens. Immunology 82, 365–369 (1994).
Modigliani, Y. et al. Establishment of tissue-specific tolerance is driven by regulatory T cells selected by thymic epithelium. Eur. J. Immunol. 26, 1807–1815 (1996).
Thorstenson, K. M. & Khoruts, A. Generation of anergic CD25+CD4+ T cells with immunoregulatory potential in vivo following induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167, 188–195 (2001).
O'Garra, A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8, 275–283 (1998).
Lanzavecchia, A. & Sallusto, F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science 290, 92–97 (2000).
Steinbrink, K., Wolfl, M., Jonuleit, H., Knop, J. & Enk, A. H. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 159, 4772–4780 (1997).
Jonuleit, H., Schmitt, E., Schuler, G., Knop, J. & Enk, A. H. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192, 1213–1222 (2000).
Dhodapkar, M. V., Steinman, R. M., Krasovsky, J., Munz, C. & Bhardwaj, N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193, 233–238 (2001).
Yamagiwa, S., Gray, J. D., Hashimoto, S. & Horwitz, D. A. A role for TGF-β in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J. Immunol. 166, 7282–7289 (2001).
Hoyne, G. F. et al. Serrate1-induced notch signalling regulates the decision between immunity and tolerance made by peripheral CD4+ T cells. Int. Immunol. 12, 177–185 (2000).
Powrie, F., Carlino, J., Leach, M. W., Mauze, S. & Coffman, R. L. A critical role for transforming growth factor-β but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RBlo CD4+ T cells. J. Exp. Med. 183, 2669–2674 (1996).
Asseman, C., Mauze, S., Leach, M. W., Coffman, R. L. & Powrie, F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190, 995–1004 (1999).
Ding, L. & Shevach, E. M. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J. Immunol. 148, 3133–3139 (1992).
Fiorentino, D. F. et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146, 3444–3451 (1991).
Takeuchi, M., Alard, P. & Streilein, J. W. TGF-β promotes immune deviation by altering accessory signals of antigen-presenting cells. J. Immunol. 160, 1589–1597 (1998).
Kitani, A. et al. Treatment of experimental (Trinitrobenzene sulfonic acid) colitis by intranasal administration of transforming growth factor (TGF)-β1 plasmid: TGF-β1-mediated suppression of T helper cell type 1 response occurs by interleukin (IL)-10 induction and IL-12 receptor β2 chain downregulation. J. Exp. Med. 192, 41–52 (2000).
Letterio, J. J. & Roberts, A. B. Regulation of immune responses by TGF-β. Annu. Rev. Immunol. 16, 137–161 (1998).
Gorelik, L. & Flavell, R. A. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12, 171–181 (2000).
Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O'Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 (2001).
Takeda, K. et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10, 39–49 (1999).
Thornton, A. M. & Shevach, E. M. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164, 183–190 (2000).
Cederbom, L., Hall, H. & Ivars, F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur. J. Immunol. 30, 1538–1543 (2000).
Shevach, E. M. Certified Professionals. CD4+CD25+ suppressor T cells. J. Exp. Med. 193, 41–46 (2001).
Suri-Payer, E. & Cantor, H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by cd4(+)cd25(+)t cells. J. Autoimmunity 16, 115–123 (2001).
Lepault, F. & Gagnerault, M. C. Characterization of peripheral regulatory CD4+ T cells that prevent diabetes onset in nonobese diabetic mice. J. Immunol. 164, 240–247 (2000).
Chambers, C. A., Kuhns, M. S., Egen, J. G. & Allison, J. P. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 19, 565–594 (2001).
Chen, W., Jin, W. & Wahl, S. M. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor β (TGF-β) production by murine CD4+ T cells. J. Exp. Med. 188, 1849–1857 (1998).
Malmstrom, V. et al. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored scid mice. J. Immunol. 166, 6972–6981 (2001).
Scheerens, H., Hessel, E., de Waal-Malefyt, R., Leach, M. W. & Rennick, D. Characterization of chemokines and chemokine receptors in two murine models of inflammatory bowel disease: IL-10−/− mice and Rag-2−/− mice reconstituted with CD4+CD45RBhi T cells. Eur. J. Immunol. 31, 1465–1474 (2001).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Medzhitov, R. & Janeway, C. A. Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell 91, 295–298 (1997).
Mackay, C. R. Homing of naive, memory and effector lymphocytes. Curr. Opin. Immunol. 5, 423–427 (1993).
Randolph, G. J., Beaulieu, S., Lebecque, S., Steinman, R. M. & Muller, W. A. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282, 480–483 (1998).
Albert, M. L. et al. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188, 1359–1368 (1998).
Sauter, B. et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191, 423–434 (2000).
Kurts, C. et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 184, 923–930 (1996).
Forster, I. & Lieberam, I. Peripheral tolerance of CD4 T cells following local activation in adolescent mice. Eur. J. Immunol. 26, 3194–3202 (1996).
Adler, A. J. et al. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J. Exp. Med. 187, 1555–1564 (1998).
Banchereau, J. et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 (2000).
Taguchi, O. & Takahashi, T. Administration of anti-interleukin-2 receptorα antibody in vivo induces localized autoimmune disease. Eur. J. Immunol. 26, 1608–1612 (1996).
Acknowledgements
We thank O. Annacker, S. Read, D. Mason, L. Stephens and A. Gallimore for numerous helpful discussions and critical review of the manuscript. K. M. and F. P. are supported by the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maloy, K., Powrie, F. Regulatory T cells in the control of immune pathology. Nat Immunol 2, 816–822 (2001). https://doi.org/10.1038/ni0901-816
Issue Date:
DOI: https://doi.org/10.1038/ni0901-816
This article is cited by
-
Increased Nasal Blimp1 + Treg Cells After Sublingual Immunotherapy Reflect the Efficacy of Treatment in Allergic Rhinitis
Advances in Therapy (2024)
-
Ossifying fibrous epulis as an IgG4-related disease of the oral cavity: a case report and literature review
BMC Oral Health (2022)
-
The role of gut microbiota in the development of colorectal cancer: a review
International Journal of Colorectal Disease (2022)
-
K2P18.1 translates T cell receptor signals into thymic regulatory T cell development
Cell Research (2022)
-
Gut priming with bovine colostrum and T regulatory cells in preterm neonates: a randomized controlled trial
Pediatric Research (2021)