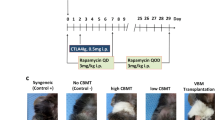
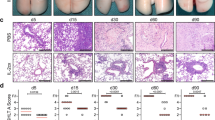
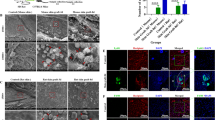
Abstract
Secondary lymphoid organs (the spleen, lymph nodes and mucosal lymphoid tissues) provide the proper environment for antigen-presenting cells to interact with and activate naive T and B lymphocytes1. Although it is generally accepted that secondary lymphoid organs are essential for initiating immune responses to microbial antigens and to skin allografts2,3,4,5,6, the prevailing view has been that the immune response to primarily vascularized organ transplants such as hearts and kidneys does not require the presence of secondary lymphoid tissue. The assumption has been that the immune response to such organs is initiated in the graft itself when recipient lymphocytes encounter foreign histocompatibility antigens presented by the graft's endothelial cells7,8,9,10,11,12,13. In contrast to this view, we show here that cardiac allografts are accepted indefinitely in recipient mice that lack secondary lymphoid tissue, indicating that the alloimmune response to a vascularized organ transplant cannot be initiated in the graft itself. Moreover, we demonstrate that the permanent acceptance of these grafts is not due to tolerance but is because of immunologic ‘ignorance’.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Picker, L.J. & Siegelman, M.H. in Fundamental Immunology 4th edn. (ed. Paul, W.E.) 479–531 (Lippincott-Raven, New York, 1999).
Goodnow, C.C. Chance encounters and organized rendezvous. Immunol. Rev. 156, 5–10 (1997).
Zinkernagel, R.M. et al. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunological Reviews 156, 199–209 (1997).
Karrer, U. et al. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11−/−) mutant mice. J. Exp. Med. 185, 2157–2170 (1997).
Barker, C.F. & Billingham, R.E. The role of afferent lymphatics in the rejection of skin homografts. J. Exp. Med. 128, 197–221 (1968).
Miyawaki, S. et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur. J. Immunol. 24, 429–434 (1994).
Medawar, P.B. The homograft reaction. Proc. R. Soc. Lond. B 149, 145–148 (1957).
Strober, S. & Gowans, J.L. The role of lymphocytes in the sensitization of rats to renal homografts. J. Exp. Med. 122, 347–360 (1965).
Brent, L. & Medawar, P.B. Cellular immunity and the homograft reaction. Br. Med. Bull. 23, 55–60 (1967).
Vetto, R.M. & Lawson, R.K. The role of vascular endothelium in the afferent pathway as suggested by the alymphatic renal homotransplant. Transplantation 5, 1537–1539 (1967).
Pedersen, N.C. & Morris, B. The role of the lymphatic system in the rejection of homografts: A study of lymph from renal transplants. J. Exp. Med. 133, 936–969 (1970).
Pober, J.S., Orosz, C.G., Rose, M.L. & Savage, C.O.S. Can graft endothelial cells initiate a host anti-graft immune response? Transplantation 61, 343–349 (1996).
Briscoe, D.M., Alexander, S.I. & Lichtman, A.H. Interactions between T lymphocytes and endothelial cells in allograft rejection. Curr. Opin. Immunol. 10, 525–531 (1998).
Roberts, C.W.M., Shutter, J.R. & Korsmeyer, S.J. Hox11 controls the genesis of the spleen. Nature 368, 747–749 (1994).
Shinkura, R. et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-κb-inducing kinase. Nature Genet. 22, 74–77 (1999).
Mombaerts, P., Arnoli, J., Russ, F., Tonegawa, S. & Kaufmann, S.H.E. Different roles ofα-β and γ-δ T cells in immunity against an intracellular bacterial pathogen. Nature 365, 53–56 (1993).
Ochsenbein, A.F. et al. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc. Natl. Acad. Sci. USA 96, 2233–2238 (1999).
Goes, N. et al. Disturbed MHC regulation in interferon-γ knockout mouse. J. Immunol. 155, 4559–4566 (1995).
Grewal, I.S. & Flavell, R.A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immuno. 16, 111–135 (1998).
Ma, W. & Pober, J.S. Human endothelial cells effectively costimulate cytokine production by, but not differentiation of, naive CD4+ T cells. J. Immunol. 161, 2158–2167 (1998).
Perez, V.L., Henault, L. & Lichtman, A.H. Endothelial antigen presentation: stimulation of previously activated but not naive TCR-transgenic mouse cells. Cell. Immunol. 189, 31–40 (1998).
Corry, R.J., Winn, H.J. & Russel, P.S. Primarily vascularized allografts of hearts in mice: The role of H-2D, H-2K, and non H-2 antigens. Transplantation 16, 343–350 (1973).
Konieczny, B.T. et al. IFNγ is critical for long term allograft survival induced by blocking the CD28 and CD40L T cell costimulation pathways. J. Immunol. 160, 2059–2064 (1998).
Acknowledgements
We thank F.K. Baddoura for the histopathologic analyses and C.P. Larsen, R. Ahmed, D. Briscoe and J. S. Pober for discussions. This work was supported by National Institutes of Health grants AI41643 (F.G.L.) and AI44644 (F.G.L.), and by the Carlos and Marguerite Mason Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakkis, F., Arakelov, A., Konieczny, B. et al. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med 6, 686–688 (2000). https://doi.org/10.1038/76267
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/76267
This article is cited by
-
Tacrolimus-Eluting Disk within the Allograft Enables Vascularized Composite Allograft Survival with Site-Specific Immunosuppression without Systemic Toxicity
Pharmaceutical Research (2022)
-
The role of lymphangiogenesis in cardiovascular diseases and heart transplantation
Heart Failure Reviews (2022)
-
Application of exosomes as liquid biopsy in clinical diagnosis
Signal Transduction and Targeted Therapy (2020)
-
Memory T cells in organ transplantation: progress and challenges
Nature Reviews Nephrology (2016)
-
The CD8 T‐cell response during tolerance induction in liver transplantation
Clinical & Translational Immunology (2016)