Abstract
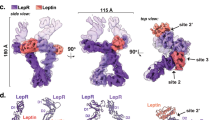
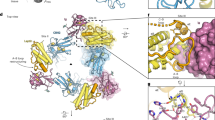
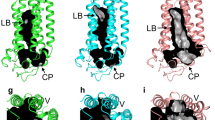
Mutations in the obese gene (OB) or in the gene encoding the OB receptor(OB-R) result in obesity, infertility and diabetes in a variety of mouse phenotypes1–7. The demonstration that OB protein (also known as leptin) can normalize body weight in ob/ob mice has generated enormous interest8–11. Most human obesity does not appear to result from a mutant form of leptin: rather, serum leptin concentrations are increased and there is an apparent inability to transport it to the central nervous system (CNS)12. Injection of leptin into the CNS of overfed rodents resistant to peripheral administration was found to induce biological activity13. Consequently, for the leptin to act as a weight-lowering hormone in human obesity, it appears that appropriate concentrations must be present in the CNS. This places a premium on understanding the structure of the hormone in order to design more potent and selective agonists. Here we report the crystal structure at 2.4 Å resolution of a human mutant OB protein (leptin-E100) that has comparable biological activity to wild type but which crystallizes more readily. The structure reveals a four-helix bundle similar to that of the long-chain helical cytokine family14.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
1. Coleman, D. L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14, 141-148 (1978). 2. Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425-432 (1994). 3. Cioffi, J. A. et al. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nature Med. 2, 585-589 (1996). 4. Chehab, F. F., Mounzih, K., Lu, R. & Lim, M. E. Early onset of reproductive function in normal female mice treated with leptin. Science 275, 88-90 (1996). 5. Ahima, R. S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382, 250-252 (1996). 6. Tartaglia, L. A. et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 83, 1263-1271 (1995). 7. Lee, G.-H. et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379, 632-635 (1996). 8. Pelleymounter, M. A. et al. Effects of the obese gene product on body weight regulation on ob/ob mice. Science 269, 540-543 (1995). 9. Halaas, J. L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543-546 (1995). 10. Campfield, L. A. et al. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546-549 (1995). 11. Stephens, T. W. et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377, 530-532 (1996). 12. Caro, J. F. et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348, 159-161 (1996). 13. Heek, M. V. et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. /. Clin. Invest. 99, 385-390 (1997). 14. Sprang, S. R. & Bazan, J. F. Cytokine structural taxonomy and mechanisms of receptor engagement. Curr. Opin. Struct. Biol. 3, 815-827 (1993). 15. Madej, T., Boguski, M. S. & Bryant, S. H. Threading analysis suggests that the obese gene product may be a helical cytokine. FEES Lett. 373, 13-18 (1995). 16. Hill, C. P., Osslund, T. D. & Eisenberg, D. The structure of granulocyte-colony-stimulating factor and its relationship to other growth factors. Proc. Natl Acad. Sci. USA 90, 5176-5171 (1993). 17. Robinson, R. C. et al. The crystal structure and biological function of leukemia inhibitory factor: implication for receptor binding. Cell 77, 1101-1116 (1994). 18. McDonald, N. Q., Panayotatos, N. & Hendrickson, W. A. Crystal structure of dimeric human ciliary neurotrophic factor determined by MAD phasing. EMBOJ. 14, 2689-2699 (1995). 19. De Vos, A. M., Ultsch, M. & Kossiakoff, A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255, 306-312 (1992). 20. Taniguchi, T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science 268, 251-255 (1995). 21. Vaisse, C. et al. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nature Genet. 14, 95-97 (1996). 22. Ghilardi, N. etal. Defective STAT signaling by the leptin receptor in diabetic mice. Proc. Natl Acad. Sci. USA 93, 6231-6235(1996). 23. Rozwarski, D. A. et al. Structural comparisons among the short-chain helical cytokines. Structure 2, 159-173 (1994). 24. Xu, G.-Y. et al. Complete 'H, l5N and 13C assignments, secondary structure, and topology of recombinant human interleukin-6./. Biomolecular NMR 8, 123-135 (1996). 25. Heldin, C.-H. Dimerization of cell surface receptors in signal transduction. Cell 80, 213-223 (1995). 26. Otwinowski, Z. Oscillation data reduction program. Proc. CCP4 Study Weekend 29-30, 56-62 (1993). 27. Collaborative Computing Project, No. 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760-763 (1994). 28. Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgarrd, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110-119 (1991). 29. Brunger, A. T. XPLOR Version 3.1: A System for X-ray Crystallography and NMR (Yale Univ. Press, New Haven, CT, 1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, F., Basinski, M., Beals, J. et al. Crystal structure of the obese protein Ieptin-E100. Nature 387, 206–209 (1997). https://doi.org/10.1038/387206a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/387206a0
This article is cited by
-
TRP channels associated with macrophages as targets for the treatment of obese asthma
Lipids in Health and Disease (2024)
-
Unexpected identification of obesity-associated mutations in LEP and MC4R genes in patients with anorexia nervosa
Scientific Reports (2024)
-
Associations between chronic widespread pain, pressure pain thresholds, leptin, and metabolic factors in individuals with knee pain
BMC Musculoskeletal Disorders (2023)
-
Breast feeding, obesity, and asthma association: clinical and molecular views
Clinical and Molecular Allergy (2023)
-
Chemical shift assignments of wildtype human leptin
Biomolecular NMR Assignments (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.