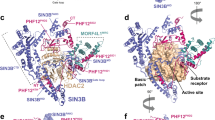
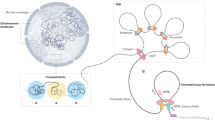
Abstract
Steroid receptors and coactivator proteins are thought to stimulate gene expression by facilitating the assembly of basal transcription factors into a stable preinitiation complex1. What is not clear, however, is how these transcription factors gain access to transcriptionally repressed chromatin to modulate the transactivation of specific gene networks in vivo. The available evidence indicates that acetylation of chromatin in vivo is coupled to transcription and that specific histone acetyltransferases (HATs)target histones bound to DNA and overcome the inhibitory effect of chromatin on gene expression2,3,4. The steroid-receptor coactivator SRC-1 is a coactivator for many members of the steroid-hormone receptor superfamily of ligand-inducible transcription factors5. Here we show that SRC-1 possesses intrinsic histone acetyltransferase activity and that it also interacts with another HAT, p300/CBP-associated factor (PCAF). The HAT activity of SRC-1 maps to its carboxy-terminal region and is primarily specific for histones H3 and H4. Acetylation by SRC-1 and PCAF of histones bound at specific promoters may result from ligand binding to steroid receptors and could be a mechanism by which the activation functions of steroid receptors and associated coactivators enhance formation of a stable preinitiation complex, thereby increasing transcription of specific genes from transcriptionally repressed chromatin templates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Klein-Hitpass, L. et al. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell 60, 247–257 (1990).
Brownell, J. E. & Allis, C. D. Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr. Opin. Genet. Dev. 6, 176–184 (1996).
Wolffe, A. P. & Pruss, D. Targeting chromatin disruption: transcription regulators that acetylate histones. Cell 86, 817–819 (1996).
Wade, P. A. & Wolffe, A. P. Histone acetyltransferases in control. Curr. Biol. 7, R82–R84 (1997).
Onate, S. A., Tsai, S. Y., Tsai, M.-J. & O'Malley, B. W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270, 1354–1357 (1995).
Yang, X.-J. et al. Ap300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382, 319–324 (1996).
Bannister, A. J. & Kouzarides, T. The CBP co-activator is a histone acetyltransferase. Nature 384, 641–643 (1996).
Ogryzko, V. V. et al. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87, 953–959 (1996).
Mizzen, C. A. et al. The TAFII250 subunit of TFIID has histone acetyltransferase activity. Cell 87, 1261–1270 (1996).
Brownell, J. E. et al. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84, 843–852 (1996).
Candau, R., Zhou, J. X., Allis, C. D. & Berger, S. L. Histone accetyltransferase activity and interaction with ADA2 are critical for GCN5 function in vivo. EMBO J. 16, 555–565 (1997).
Archer, T. K., Lefebvre, P., Wolford, R. D. & Hager, G. L. Transcription factor loading on the MMTV promoter: a bimodal mechanism for promoter activation. Science 255, 1573–1576 (1992).
Truss, M. et al. Hormone induces binding of receptors and transcription factors to a rearranged nucleosome on the MMTV promoter in vivo. EMBO J. 14, 1737–1751 (1995).
Felsenfeld, G. et al. Chromatin structure and gene expression. Proc. Natl Acad. Sci. USA 93, 9384–9388 (1996).
Wong, J., Shi, Y-B. & Wolffe, A. P. Arole for nucleosome assembly in both silencing and activation of the Xenopus TRβA gene by the thyroid hormone receptor. Genes Dev. 9, 2696–2711 (1995).
Wolffe, A. P. Nucleosome positioning and modification: chromatin structures that potentiate transcription. Trends Biochem. Sci. 9, 240–244 (1994).
Jenster, G. et al. Steroid receptor induction of gene transcription: a two-step model. Proc. Natl Acad. Sci. USA 94, 7879–7884 (1997).
Kamei, Y. et al. ACBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85, 403–414 (1996).
Smith, C. S., Onate, S. A., Tsai, M.-J. & O'Malley, B. W. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc. Natl Acad. Sci. USA 93, 8884–8888 (1996).
Wong, J., Shi, Y-B. & Wolffe, A. P. Determinants of chromatin disruption and transcriptional regulation instigated by the thyroid hormone receptor: hormone regulated chromatin disruption is not sufficient for transcriptional activation. EMBO J. 11, 3158–3171 (1997).
Wolffe, A. P. Histone deacetylase: a regulator of transcription. Science 272, 371–372 (1996).
Taunton, J., Hassig, C. A. & Schreiber, S. L. Amammalian histone deacetylase related to a yeast transcriptional regulator Rpd3. Science 272, 408–411 (1996).
Kingston, R. E., Bunker, C. A. & Imbalzano, A. M. Repression and activation by multiprotein complexes that alter chromatin structure. Genes Dev. 10, 950–920 (1996).
Baniahmad, A. et al. Interaction of human thyroid hormone receptor beta with transcription factor TFIIB may mediate target gene derepression and activation by thyroid hormone. Proc. Natl Acad. Sci. USA 90, 8832–8836 (1993).
Tam, J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc. Natl Acad. Sci. USA 85, 5409–5413 (1988).
Brownell, J. E. & Allis, C. D. An activity gel assay detects a single, catalytically active histone acetyltransferase subunit in Tetrahymena macronuclei. Proc. Natl Acad. Sci. USA 92, 6364–6368 (1995).
Acknowledgements
We thank P. Samora and L. Gong for technical support of the experiments; D. P. Edwards for assistance with monoclonal antibody production; Y. Nakatani for the human Flag-PCAF plasmid; and A. Wolffe and J. Wong for discussion. This work was supported by an NIH NRSA postdoctoral fellowship (T.E.S.), a TALENT stipend from the Netherlands Organization for Scientific Research (G.J.) and an NIH grant (B.W.O.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spencer, T., Jenster, G., Burcin, M. et al. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389, 194–198 (1997). https://doi.org/10.1038/38304
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/38304
This article is cited by
-
The multifaceted therapeutic value of targeting steroid receptor coactivator-1 in tumorigenesis
Cell & Bioscience (2024)
-
Natural compounds targeting nuclear receptors for effective cancer therapy
Cancer and Metastasis Reviews (2023)
-
KAT2A-mediated AR translocation into nucleus promotes abiraterone-resistance in castration-resistant prostate cancer
Cell Death & Disease (2021)
-
Oxycodone self-administration activates the mitogen-activated protein kinase/ mitogen- and stress-activated protein kinase (MAPK-MSK) signaling pathway in the rat dorsal striatum
Scientific Reports (2021)
-
Unraveling the epigenetic landscape of glomerular cells in kidney disease
Journal of Molecular Medicine (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.