Key Points
-
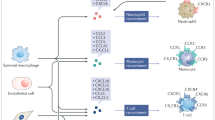
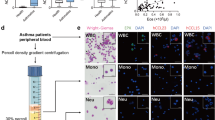
Asthma is now thought to be an inflammatory disease, the severity of which is linked to the intensity of leukocyte recruitment into the lung. Chemokines are small cytokines that regulate the number, intensity and activation of leukocyte migration into the asthmatic airways. A number of chemokines have been identified in the airways during human asthmatic responses.
-
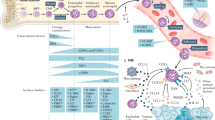
Chemokines in two distinct families (CxC and CC) now number upwards of ∼45 different members which have diverse and distinct functions during homeostasis and disease progression.
-
Six CxC and ten CC chemokine G-protein-coupled receptors have been identified which engage a number of chemokine ligands in a promiscuous manner, causing confusion as well as diverse options for therapeutic intervention.
-
It now appears that TH2 lymphocytes and eosinophils are the most important cell types that are associated with development of severe asthmatic disease. Identification of specific chemokines and receptors that are responsible for the migration of these cell populations into the airways are the focus of most discovery programs.
-
Animal models of asthma have identified a number of viable chemokine and chemokine receptor targets, the inhibition of which might substantially alter asthmatic disease progression.
-
Chemokines are important factors for directing specific leukocyte recruitment and activation, including degranulation of eosinophils, mast cells and neutrophils, as well as having the ability to direct the immune response. All of these aspects will need to be considered when investigating new methods for inhibiting chemokines to modulate disease progression.
Abstract
The prevalence of asthma has risen drastically in the last two decades, with a worldwide impact on health care systems. Although several factors contribute to the development of asthma, inflammation seems to be a common factor that leads to the most severe asthmatic responses. In the past decade, researchers have characterized a large group of chemotactic cytokines, also known as chemokines, which are implicated in asthmatic inflammation. These chemokines control and direct the migration and activation of various leukocyte populations. Targeting chemokines should lead to new ways of controlling the inflammatory asthmatic response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hartert, T. V. & Peebles, R. S. Epidemiology of asthma: the year in review. Curr. Opin. Pulm. Med. 6, 4–9 (2000).
Marwick, C. Helping city children control asthma. JAMA 277, 1503–1504 (1997).
Schuh, S., Johnson, D., Stephens, D., Callahan, S. & Canny, G. Hospitalization patterns in severe acute asthma in children. Pediatr. Pulmonol. 23, 184–192 (1997).
Kon, O. M. & Kay, A. B. T cells and chronic asthma. Int. Arch. Allergy Immunol. 118, 133–135 (1999).
Kay, A. B. Pathology of mild, severe, and fatal asthma. Am. J. Respir. Crit. Care Med. 154, S66–S69 (1996).
Mapp, C., Boschetto, P., Miotto, D., De Rosa, E. & Fabbri, L. M. Mechanisms of occupational asthma. Annu. Allergy Asthma Immunol. 83, 645–664 (1999). | PubMed |
Bentley, A. M., Kay, A. B. & Durham, S. R. Human late asthmatic reactions. Clin. Exp. Allergy 27, 71–86 (1997).
Muro, S., Minshall, E. M. & Hamid, Q. A. The pathology of chronic asthma. Clin. Chest Med. 21, 225–244 (2000).
Busse, W. W., Banks-Schlegel, S. & Wenzel, S. E. Pathophysiology of severe asthma. J. Allergy Clin. Immunol. 106, 1033–1042 (2000).
Rossi, D. & Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18, 217–242 (2000).
Zlotnik, A. & Yoshie, O. Chemokines: a new classification system and their role in immunity. Immunity 12, 121–127 (2000).Outlines the new nomenclature for the chemokines, which clarifies this area of confusion. The table and charts clearly identify the order and specific receptor interactions.
Sotsios, Y. & Ward, S. G. Phosphoinositide 3-kinase: a key biochemical signal for cell migration in response to chemokines. Immunol. Rev. 177, 217–235 (2000).
Lukacs, N. W., Oliveira, S. H. & Hogaboam, C. M. Chemokines and asthma: redundancy of function or a coordinated effort? J. Clin. Invest. 104, 995–999 (1999).
Powell, N. et al. Increased expression of mRNA encoding RANTES and MCP-3 in the bronchial mucosa in atopic asthma. Eur. Respir. J. 9, 2454–2460 (1996).
Stellato, C. et al. Production of the novel C-C chemokine MCP-4 by airway cells and comparison of its biological activity to other C-C chemokines. J. Clin. Invest. 99, 926–936 (1997).
Lamkhioued, B. et al. Monocyte chemoattractant protein (MCP)-4 expression in the airways of patients with asthma. Induction in epithelial cells and mononuclear cells by proinflammatory cytokines. Am. J. Respir. Crit. Care Med. 162, 723–732 (2000).
Taha, R. A. et al. Eotaxin and monocyte chemotactic protein-4 mRNA expression in small airways of asthmatic and nonasthmatic individuals. J. Allergy Clin. Immunol. 103, 476–483 (1999).
Berkman, N. et al. Expression of RANTES mRNA and protein in airways of patients with mild asthma. Am. J. Respir. Crit. Care Med. 154, 1804–1811 (1996).
Wang, J. H., Devalia, J. L., Xia, C., Sapsford, R. J. & Davies, R. J. Expression of RANTES by human bronchial epithelial cells in vitro and in vivo and the effect of corticosteroids. Am. J. Respir. Cell. Mol. Biol. 14, 27–35 (1996).
Sallusto, F., Lanzavecchia, A. & Mackay, C. R. Chemokines and chemokine receptors in T-cell priming and TH1/TH2-mediated responses. Immunol. Today 19, 568–574 (1998).
Sallusto, F., Lenig, D., Mackay, C. R. & Lanzavecchia, A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187, 875–883 (1998).
Bonecchi, R. et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (TH1s) and TH2s. J. Exp. Med. 187, 129–134 (1998).References 21 and 22 are the initial studies that identified the preferential expression of specific chemokine receptors on polarized T lymphocytes. They outline the basic principle of preferential expression on the basis of a specific cytokine environment.
Sallusto, F., Mackay, C. R. & Lanzavecchia, A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science 277, 2005–2007 (1997).
Uguccioni, M. et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J. Clin. Invest. 100, 1137–1143 (1997).
Alam, R. et al. Increased MCP-1, RANTES, and MIP-1α in bronchoalveolar lavage fluid of allergic asthmatic patients. Am. J. Respir. Crit. Care Med. 153, 1398–1404 (1996).
Sousa, A. R. et al. Increased expression of the monocyte chemoattractant protein-1 in bronchial tissue from asthmatic subjects. Am. J. Respir. Cell Mol. Biol. 10, 142–147 (1994).
Tillie-Leblond, I. et al. CC chemokines and interleukin-5 in bronchial lavage fluid from patients with status asthmaticus. Potential implication in eosinophil recruitment. Am. J. Respir. Crit. Care Med. 162, 586–592 (2000).
Collins, P. D., Marleau, S., Griffiths-Johnson, D. A., Jose, P. J. & Williams, T. J. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J. Exp. Med. 182, 1169–1174 (1995).
Warringa, R. A. et al. Modulation of eosinophil chemotaxis by interleukin-5. Am. J. Respir. Cell Mol. Biol. 7, 631–636 (1992).
Corrigan, C. J. & Kay, A. B. T cells and eosinophils in the pathogenesis of asthma. Immunol. Today 13, 501–507 (1992).
Kapp, A., Zeck-Kapp, G., Czech, W. & Schopf, E. The chemokine RANTES is more than a chemoattractant: characterization of its effect on human eosinophil oxidative metabolism and morphology in comparison with IL-5 and GM-CSF. J. Invest. Dermatol. 102, 906–914 (1994).
Meurer, R. et al. Formation of eosinophilic and monocytic intradermal inflammatory sites in the dog by injection of human RANTES but not human monocyte chemoattractant protein 1, human macrophage inflammatory protein 1α, or human interleukin 8. J. Exp. Med. 178, 1913–1921 (1993).
Heath, H. et al. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J. Clin. Invest. 99, 178–184 (1997).
Gao, J. L. et al. Identification of a mouse eosinophil receptor for the CC chemokine eotaxin. Biochem. Biophys. Res. Commun. 223, 679–684 (1996).
Ponath, P. D. et al. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J. Exp. Med. 183, 2437–2448 (1996).
Combadiere, C., Ahuja, S. K. & Murphy, P. M. Cloning and functional expression of a human eosinophil CC chemokine receptor. J. Biol. Chem. 271, 11034 (1996).
Daugherty, B. L. et al. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J. Exp. Med. 183, 2349–2354 (1996).
Ponath, P. D. et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J. Clin. Invest. 97, 604–612 (1996).
Jose, P. J. et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J. Exp. Med. 179, 881–887 (1994).A protein mediator that preferentially induced eosinophil accumulation in the airway was first discovered in guinea-pig airways, but the importance for human disease was quickly realized. Much of the early therapy in the chemokine field has been geared towards inhibiting the action of eotaxin with its specific receptor, CCR3.
Kitaura, M. et al. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J. Biol. Chem. 271, 7725–7730 (1996).
Rothenberg, M. E., Luster, A. D. & Leder, P. Murine eotaxin: an eosinophil chemoattractant inducible in endothelial cells and in interleukin 4-induced tumor suppression. Proc. Natl Acad. Sci. USA 92, 8960–8964 (1995).
Yang, Y., Loy, J., Ryseck, R. P., Carrasco, D. & Bravo, R. Antigen-induced eosinophilic lung inflammation develops in mice deficient in chemokine eotaxin. Blood 92, 3912–3923 (1998).
Rothenberg, M. E., MacLean, J. A., Pearlman, E., Luster, A. D. & Leder, P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J. Exp. Med. 185, 785–790 (1997).The finding that eotaxin-deficient mice showed virtually normal allergic airway responses allowed investigators to realize that targeting the receptor, CCR3, might be more efficacious than an individual chemokine ligand.
Fujisawa, T. et al. Chemokines induce eosinophil degranulation through CCR-3. J. Allergy Clin. Immunol. 106, 507–513 (2000).
Bates, M. E., Green, V. L. & Bertics, P. J. ERK1 and ERK2 activation by chemotactic factors in human eosinophils is interleukin 5-dependent and contributes to leukotriene C4 biosynthesis. J. Biol. Chem. 275, 10968–10975 (2000).
Kampen, G. T. et al. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood 95, 1911–1917 (2000).
Rot, A. et al. RANTES and macrophage inflammatory protein 1α induce the migration and activation of normal human eosinophil granulocytes. J. Exp. Med. 176, 1489–1495 (1992).
Lukacs, N. W. et al. Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C-C family chemokines in allergic airway inflammation. J. Immunol. 158, 4398–4404 (1997).
Lukacs, N. W., Strieter, R. M., Shaklee, C. L., Chensue, S. W. & Kunkel, S. L. Macrophage inflammatory protein-1α influences eosinophil recruitment in antigen-specific airway inflammation. Eur. J. Immunol. 25, 245–251 (1995).
Lukacs, N. W. et al. C-C chemokine-induced eosinophil chemotaxis during allergic airway inflammation. J. Leukoc. Biol. 60, 573–578 (1996).
Gonzalo, J. A. et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 188, 157–167 (1998).One of the earliest studies showing that chemokines were not just a family of cytokines with overlapping functions, but rather induced factors that participated in a response in a coordinated manner. Multiple chemokines have a significant role in different aspects of the allergen-induced responses in the lung.
Sabroe, I. et al. Differential regulation of eosinophil chemokine signaling via CCR3 and non-CCR3 pathways. J. Immunol. 162, 2946–2955 (1999).
Bochner, B. S. et al. Macrophage-derived chemokine induces human eosinophil chemotaxis in a CC chemokine receptor 3- and CC chemokine receptor 4-independent manner. J. Allergy Clin. Immunol. 103, 527–532 (1999).
Gonzalo, J. A. et al. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 163, 403–411 (1999).
Chvatchko, Y. et al. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J. Exp. Med. 191, 1755–1764 (2000).
Mantovani, A., Gray, P. A., Van Damme, J. & Sozzani, S. Macrophage-derived chemokine (MDC). J. Leukoc. Biol. 68, 400–404 (2000).
Matsukawa, A. et al. Pivotal role of the CC chemokine, macrophage-derived chemokine, in the innate immune response. J. Immunol. 164, 5362–5368 (2000).
Campbell, E. M., Kunkel, S. L., Strieter, R. M. & Lukacs, N. W. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J. Immunol. 161, 7047–7053 (1998).
Sekiya, T. et al. Inducible expression of a TH2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J. Immunol. 165, 2205–2213 (2000).
Andrew, D. P. et al. STCP-1 (MDC) CC chemokine acts specifically on chronically activated TH2 lymphocytes and is produced by monocytes on stimulation with TH2 cytokines IL-4 and IL-13. J. Immunol. 161, 5027–5038 (1998).
Mochizuki, M., Bartels, J., Mallet, A. I., Christophers, E. & Schroder, J. M. IL-4 induces eotaxin: a possible mechanism of selective eosinophil recruitment in helminth infection and atopy. J. Immunol. 160, 60–68 (1998).
Li, L., Xia, Y., Nguyen, A., Feng, L. & Lo, D. TH2-induced eotaxin expression and eosinophilia coexist with TH1 responses at the effector stage of lung inflammation. J. Immunol. 161, 3128–3135 (1998).
Zhang, S., Lukacs, N. W., Lawless, V. A., Kunkel, S. L. & Kaplan, M. H. Cutting edge: differential expression of chemokines in TH1 and TH2 cells is dependent on Stat6 but not Stat4. J. Immunol. 165, 10–14 (2000).Identifies a primary role for T H 2 cytokine-induced STAT6 regulation of chemokines and their receptors on T lymphocytes, indicating that their expression might be dependent on gene regulation and not on differentiation state.
Mathew, A. et al. Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation. J. Exp. Med. 193, 1087–1096 (2001).
Goebeler, M. et al. Interleukin-13 selectively induces monocyte chemoattractant protein-1 synthesis and secretion by human endothelial cells. Involvement of IL-4Rα and Stat6 phosphorylation. Immunology 91, 450–457 (1997).
Matsukura, S. et al. Interleukin-13 upregulates eotaxin expression in airway epithelial cells by a Stat6-dependent mechanism. Am. J. Respir. Cell Mol. Biol. 24, 755–761 (2001).
Hoeck, J. & Woisetschlager, M. STAT6 mediates eotaxin-1 expression in IL-4 or TNF-α-induced fibroblasts. J. Immunol. 166, 4507–4515 (2001).
Campbell, J. D. & HayGlass, K. T. T cell chemokine receptor expression in human TH1- and TH2-associated diseases. Arch. Immunol. Ther. Exp. (Warsz.) 48, 451–456 (2000).
Yoshie, O. Role of chemokines in trafficking of lymphocytes and dendritic cells. Int. J. Hematol. 72, 399–407 (2000).
Sallusto, F. & Lanzavecchia, A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 177, 134–140 (2000).This review provides an excellent focus on the coordination of T-lymphocyte and dendritic cell traffic from tissue to lymph node.
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Sallusto, F. et al. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur. J. Immunol. 29, 2037–2045 (1999).
Warnock, R. A. et al. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules. J. Exp. Med. 191, 77–88 (2000).
Tangemann, K., Gunn, M. D., Giblin, P. & Rosen, S. D. A high endothelial cell-derived chemokine induces rapid, efficient, and subset-selective arrest of rolling T lymphocytes on a reconstituted endothelial substrate. J. Immunol. 161, 6330–6337 (1998).
Stein, J. V. et al. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191, 61–76 (2000).
D'Ambrosio, D. et al. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 TH cells. J. Immunol. 161, 5111–5115 (1998).
Jagodzinski, P. P. & Trzeciak, W. H. Differential expression of chemokine receptor CXCR4 in TH1 and TH2 subtypes of CD4+ lymphocytes. Folia Histochem. Cytobiol. 38, 21–23 (2000).
Nanki, T. & Lipsky, P. E. Lack of correlation between chemokine receptor and TH1/TH2 cytokine expression by individual memory T cells. Int. Immunol. 12, 1659–1667 (2000).
Lloyd, C. M. et al. CC chemokine receptor (CCR)3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J. Exp. Med. 191, 265–274 (2000).The first study to show that different chemokine receptors are used at distinct phases of disease progression to promote T-lymphocyte accumulation. Complements the earlier studies by this group that show coordinated chemokine production.
Panina-Bordignon, P. et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J. Clin. Invest. 107, 1357–1364 (2001).
Li, L. et al. Effects of TH2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J. Immunol. 162, 2477–2487 (1999).
Saito, T. et al. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J. Infect. Dis. 175, 497–504 (1997).
Hogaboam, C. M. et al. Differential monocyte chemoattractant protein-1 and chemokine receptor 2 expression by murine lung fibroblasts derived from TH1- and TH2-type pulmonary granuloma models. J. Immunol. 163, 2193–2201 (1999).
Petering, H. et al. Detection of MCP-4 in dermal fibroblasts and its activation of the respiratory burst in human eosinophils. J. Immunol. 160, 555–558 (1998).
Ying, S. et al. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J. Immunol. 163, 6321–6329 (1999).
Gupta, S. K., Lysko, P. G., Pillarisetti, K., Ohlstein, E. & Stadel, J. M. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J. Biol. Chem. 273, 4282–4287 (1998).
Hayes, I. M. et al. Human vascular smooth muscle cells express receptors for CC chemokines. Arterioscler. Thromb. Vasc. Biol. 18, 397–403 (1998).
Crane, I. J., Wallace, C. A., McKillop-Smith, S. & Forrester, J. V. CXCR4 receptor expression on human retinal pigment epithelial cells from the blood-retina barrier leads to chemokine secretion and migration in response to stromal cell-derived factor 1α. J. Immunol. 165, 4372–4378 (2000).
Jordan, N. J. et al. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J. Clin. Invest. 104, 1061–1069 (1999).
Dwinell, M. B., Eckmann, L., Leopard, J. D., Varki, N. M. & Kagnoff, M. F. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology 117, 359–367 (1999).
Stellato, C. et al. Expression of the C-C chemokine receptor CCR3 in human airway epithelial cells. J. Immunol. 166, 1457–1461 (2001).One of the first studies to show that the chemokine receptor CCR3 can be expressed on airway epithelial cells during asthmatic responses. Interestingly, these same cells produce an abundant amount of CCR3-specific ligands during asthmatic responses. The consequences and mechanisms associated with this finding will probably have a significant impact on airway biology and physiology during disease.
Zhang, J., Lathbury, L. J. & Salamonsen, L. A. Expression of the chemokine eotaxin and its receptor, CCR3, in human endometrium. Biol. Reprod. 62, 404–411 (2000).
Oyamada, H. et al. CCR3 mRNA expression in bronchial epithelial cells and various cells in allergic inflammation. Int. Arch. Allergy Immunol. 120 (Suppl. 1), 45–47 (1999).
Porreca, E. et al. Monocyte chemotactic protein 1 (MCP-1) is a mitogen for cultured rat vascular smooth muscle cells. J. Vasc. Res. 34, 58–65 (1997).
Luo, Y., D'Amore, P. A. & Dorf, M. E. β-chemokine TCA3 binds to and activates rat vascular smooth muscle cells. J. Immunol. 157, 2143–2148 (1996).
Yue, T. L. et al. Interleukin-8. A mitogen and chemoattractant for vascular smooth muscle cells. Circ. Res. 75, 1–7 (1994).
Belperio, J. A. et al. CXC chemokines in angiogenesis. J. Leukoc. Biol. 68, 1–8 (2000).
Rossi, D. & Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18, 217–242 (2000).
Bento, A. M. & Hershenson, M. B. Airway remodeling: potential contributions of subepithelial fibrosis and airway smooth muscle hypertrophy/hyperplasia to airway narrowing in asthma. Allergy Asthma Proc. 19, 353–358. (1998).
Rollins, B. J. JE/MCP-1: an early-response gene encodes a monocyte-specific cytokine. Cancer Cells 3, 517–524 (1991).
Gharaee-Kermani, M., Denholm, E. M. & Phan, S. H. Costimulation of fibroblast collagen and transforming growth factor β1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J. Biol. Chem. 271, 17779–17784 (1996).
Blease, K. et al. Antifungal and airway remodeling roles for murine monocyte chemoattractant protein-1/CCL2 during pulmonary exposure to Asperigillus fumigatus conidia. J. Immunol. 166, 1832–1842 (2001).
Lloyd, C. M. et al. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J. Exp. Med. 185, 1371–1380 (1997).
Redington, A. E. Fibrosis and airway remodelling. Clin. Exp. Allergy 30 (Suppl. 1), 42–45 (2000).
Donohue, J. F. & Ohar, J. A. New combination therapies for asthma. Curr. Opin. Pulm. Med. 7, 62–68 (2001).
Chensue, S. W. et al. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J. Exp. Med. 193, 573–584 (2001).The first study to indicate that chemokine receptors can be targeted for therapy to alter the T H 2-specific asthmatic responses. The preferential expression of CCR8 on T H 2 cells and reduced T H 2 cytokine phenotype within the lung of CCR8-deficient mice is a vital clue for therapeutic targeting of a specific receptor.
Gonzalo, J. A. et al. Critical involvement of the chemotactic axis CXCR4/stromal cell-derived factor-1α in the inflammatory component of allergic airway disease. J. Immunol. 165, 499–508 (2000).
Zingoni, A. et al. The chemokine receptor CCR8 is preferentially expressed in TH2 but not TH1 cells. J. Immunol. 161, 547–551 (1998).
Jahnz-Rozyk, K. M., Kuna, P. & Pirozynska, E. Monocyte chemotactic and activating factor/monocyte chemoattractant protein (MCAF/MCP-1) in bronchoalveolar lavage fluid from patients with atopic asthma and chronic bronchitis. J Investig Allergol Clin Immunol 7, 254–259 (1997).
Campbell, E. M. et al. Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/− mice: the role of mast cells. J. Immunol. 163, 2160–2167 (1999).
MacLean, J. A. et al. CC chemokine receptor-2 is not essential for the development of antigen-induced pulmonary eosinophilia and airway hyperresponsiveness. J. Immunol. 165, 6568–6575 (2000).
Blease, K. et al. Enhanced pulmonary allergic responses to Aspergillus in CCR2−/− mice. J. Immunol. 165, 2603–2611 (2000).
Luther, S. A. & Cyster, J. G. Chemokines as regulators of T cell differentiation. Nature Immunol. 2, 102–107 (2001).
Acknowledgements
I thank R. Kunkel for design of the original figures.
Author information
Authors and Affiliations
Glossary
- DEGRANULATION
-
Release of mediators, proteases and so on from stored granules in the cytoplasm of mast cells, neutrophils, eosinophils or other leukocyte populations.
- AIRWAY HYPERREACTIVITY
-
Physiologic measurement of the changes in airway resistance induced by a pharmacologic stimuli, such as methacholine.
- OVA-INDUCED EOSINOPHILIA
-
Ovalbumin-induced animal model of allergic airway inflammation.
Rights and permissions
About this article
Cite this article
Lukacs, N. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol 1, 108–116 (2001). https://doi.org/10.1038/35100503
Issue Date:
DOI: https://doi.org/10.1038/35100503
This article is cited by
-
Immunoregulatory role of hesperidin against ovalbumin (OVA)-induced bronchial asthma and depression in rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Surface PEGylation suppresses pulmonary effects of CuO in allergen-induced lung inflammation
Particle and Fibre Toxicology (2019)
-
Chemokines in homeostasis and diseases
Cellular & Molecular Immunology (2018)
-
Phytochemical profiles and inhibitory effects of Tiger Milk mushroom (Lignosus rhinocerus) extract on ovalbumin-induced airway inflammation in a rodent model of asthma
BMC Complementary and Alternative Medicine (2016)
-
Sepsis induces incomplete M2 phenotype polarization in peritoneal exudate cells in mice
Journal of Intensive Care (2016)