Abstract
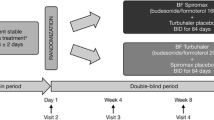
In children with asthma, twice daily administration of salmeterol 25 μg, salmeterol 50 μg and salbutamol 200 μg were compared in two, 3-month, double-blind, parallel group studies, one using metered dose inhalers (MDIs), the other using dry powder inhalers (Diskhaler, DPIs). Both studies were continued for a further 9 months during which time exacerbation rates, lung function at the clinic and adverse events were monitored. Similarities in design and methodology of the two studies justified a combined analysis. Eight hundred and forty-seven asthmatic children aged between 4 and 16 (mean 10.1) years, requiring inhaled beta2-agonist treatment were randomised to treatment. After a 2 week run-in when all bronchodilator therapy was withdrawn, 279 patients received salmeterol 25 μg bd, 290 patients salmeterol 50 μg bd and 278 patients salbutamol 200 μg bd. After 3 months' treatment the change from baseline in daily morning and evening peak expiratory flow (PEF) was significantly greater with salmeterol 50 μg bd than with salbutamol 200 μg bd (P<0.001). Salmeterol 50 μg bd was also significantly better than salmeterol 25 μg bd at improving mean morning PEF (P=0.017) but both treatments had a similar effect on evening PEF. Analysis of variance showed an interaction between baseline PEF less than 100% predicted normal value and treatment outcome. Analysis of this sub-set of patients with lower lung function revealed similar results to the total population although the improvements in PEF from baseline were greater. Data from both studies, showed that the improvement in lung function was maintained throughout 12 months' treatment. Patients receiving salmeterol 50 μg bd had significantly more symptom-free nights (P<0.01) and a higher percentage of rescue bronchodilator-free days (P=0.01). The incidence of asthma exacerbations was evenly distributed between the three treatment groups and there was no evidence of any change in the rate of occurrence of exacerbations over the 12 month period. Adverse events were no different across treatment groups or across age groups and were primarily related to the patients' disease state.
Similar content being viewed by others
Abbreviations
- CI :
-
confidence intervals
- DPI :
-
dry powder inhaler
- FEV 1 :
-
forced expiratory volume in 1 s
- MDI :
-
metered dose inhaler
- PEF :
-
peak expiratory flow
References
Britton MG, Earnshaw JS, Palmer JBD (1992) A twelve-month comparison of salmeterol with salbutamol in asthmatic patients. Eur Respir J 5: 1062–1067
Green CP, Price JF (1992) Prevention of exercise-induced asthma by inhaled salmeterol xinafoate. Arch Dis Child 67: 1014–1017
Guidelines On The Management Of Asthma (1993) Thorax 48: [Suppl] S1-S243
Kemp JP, Meltzer EO (1990) Beta2 adrenergic agonists-oral or aerosol for the treatment of asthma. J Asthma 27 (3): 149–157
Lundback B, Rawlinson DW Palmer JBD (1993) Twelve month comparison of salmeterol and salbutamol as dry powder formulations in asthmatic patients. Thorax 48: 148–153
Nasser SSM Rees PJ (1993) Theophylline. Current thoughts on the risks and beenfits of its use in asthma. Drug Safety 8 (1): 12–18
Nathan RA (1992) Beta2 agonist therapy: Oral versus inhaled delivery. J Asthma 29 (1): 49–54
Pocock SJ (1983) 3 In clinical trials. A practical approach. Wiley, Chichester
Sears MR, Taylor DR, Print CG, et al (1990) Regular inhaled beta-agonist treatment in bronchial asthma. Lancet 336: 1391–1396
Simons F, Soni N, Watson W, Becker A (1992) Bronchodilator and bronchoprotective effects of salmeterol in young patients with asthma. J Allergy Clin Immunol 90: 840–846
Spitzer WO, Suissa S, Ernst P, et al (1992) The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med 326 (8): 501–506
Stallaert R, Prins J (1991) A comparison of the metered dose inhaler and dry powder Diskhaler inhaler formulations of salmeterol in mild to moderate asthmatics. Schweiz Med Wochenschr 121 [Suppl 40/II]: 23, P2062
Van Schayk CP, Dompeling E, Herwaarden CLA van, et al (1991) Bronchodilator treatment in moderate asthma or chronic bronchitis: continuous or on demand? A randomised controlled study. BMJ 303: 1426–1431
Verberne A, Lenney W, Kerrebijn K (1991) A three-way cross-over study comparing twice daily dosing of salmeterol 25 μg and 50 μg with placebo in children with mild to moderate reversible airways disease (abstract). Am Rev Respir Dis 143: A20
Warner JO, Gotz M, Landau LI, Levison H, Milner AD, Pedersen S (1989) Management Of Asthma: A Consensus Statement. Arch Dis Child 64: 1065–1079
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Lenney, W., Pedersen, S., Boner, A.L. et al. Efficacy and safety of salmeterol in childhood asthma. Eur J Pediatr 154, 983–990 (1995). https://doi.org/10.1007/BF01958642
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01958642