Summary
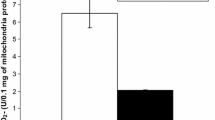
The ability of the polymorphonuclear leukocyte (PMN) oxidants, hypochlorous acid (HOC1) and hydrogen peroxide (H2O2), to oxidize proteins in rat heart and lung tissues was investigated. Cardiac myocytes, heart tissue slices, isolated perfused hearts, and lung tissue slices, were treated with HOCI and H2O2 and the extent of methionine and cysteine oxidation was determined in the cellular proteins. Cardiac tissues were found to be highly susceptible to oxidation by physiological concentrations of HOCl. For example, in isolated hearts perfused for 60 min with 100 μM HOCI, approximately 18010 of the methionine and 2801o of the cysteine residues were oxidized. Lung tissues, unlike those of the heart, were resistant to physiological concentrations of HOCI, showing no oxidation of proteins. HOCI was much more effective than H2O2 in oxidizing proteins, suggesting that HOCI may be the most reactive oxidant produced by activated PMN. These studies show that PMN oxidants, in particular HOC I, can cause significant oxidation of proteins in target tissues, and may therefore constitute a primary cause of tissue injury at sites of inflammation. In addition, these studies show that different tissues may have varying susceptibilities to PMN oxidants.
Similar content being viewed by others
References
Bulkley GB: Pathophysiology of free radical-mediated reperfusion injury. J Vase Surg 5:512–517, 1987
Halliwell B: Oxidants and human disease: some new concepts. FASEB J 1:358–364, 1987
Schmid-Schönbein GW: Capillary plugging by granulocytes and the no-reflow phenomenon in the microcirculation. Fed Proc 46:2397–2401, 1987
Harlan JM: Leukocyte-endothelial interactions. Blood 65:513–525, 1985
Mullane KM, Reed N, Salmon JA, Moncada S: Role of leucocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Therapeutics 228:510–522, 1984
Tauber AI, Babior BM: Neutrophil oxygen reduction: the enzymes and products. In: WA Pryor (ed.) Advances in Free Radical Biology and Medicine, vol. 1. Pergamon Press, New York, 1985, pp 265–307
Thomas EL, Grisham MB, Jefferson MM: Cytotoxicity of choloramines. In: G DiSabato and J Everse (eds) Methods in Enzymology, vol 132. Academic Press, New York, 1986, pp 585–593
Rinaldo JE: Mediation of ARDS by leukocytes: clinical evidence and implications for therapy. Chest 89:590–593, 1986
Tate RM, Repine JE: Neutrophils and the adult respiratory distress syndrome. Am Rev Resp Dis 128:552–559, 1983
Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE: Lung neutrophils in the adult respiratory distress syndrome: clinical and pathophysiologic significance. Am Rev Resp Dis 133:218–225, 1986
Fantone JC, Feltner DE, Brieland JK, Ward PA: Phagocytic cell-derived inflammatory mediators and lung disease. Chest 91:428–435, 1987
Janoff A, Carp H, Laurent P, Raju L: The role of oxidative processes in emphysema. Am Rev Resp Dis 127:S31-S38, 1983
Nadel JA: Inflammation and asthma. J Allergy Clin Immunol 73:651–653, 1984
Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR: Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 67:1016–1023, 1983
Lucchesi BR, Mullane KM: Leukocytes and ischemiainduced myocardial injury. In: R George, R Okun and AK Cho (eds) Annual Review of Pharmacology and Toxicology, vol 26. Annual Reviews Inc. Palo Alto 1986, pp 201–224
Clark RA: Extracellular effects of the myeloperoxidase hydrogen peroxide-halide system. In: G Weissman (ed) Advances in Inflammation Research, Vol 5. Raven Press, New York, 1983, pp 107–146
Kramer JH, Mak IT, Weglicki WB: Differential sensitivity of canine cardiac sarcolemmal and microsomal enzymes to inhibition by free radical-induced lipid peroxidation. Circ Res 55:120–124, 1984
Weiss SJ: Oxygen, ischemia and inflammation. Acta Physiol Scand 548 (Suppl):9–37, 1986
Scherer NM, Deamer DW: Oxidative stress impairs the function of sarcoplasmic reticulum by oxidation of sulfhydryl groups in the Ca2+-ATPase. Arch Biochem Biophys 246:589–601, 1986
Davies KJA, Goldberg AL: Proteins damaged by oxygen radicals are rapidly degraded in extracts of red blood cells. J Biol Chem 262:8227–8234, 1987
Fliss H, Docherty G: Oxidation of methionine in proteins of isolated rat heart myocytes and tissue slices by neutrophilgenerated oxygen free radicals. In: NS Dhalla, IR Inves and RE Beamish (eds) Myocardial Ischemia. Martinus Nijhoff, Boston, 1987, pp 85–98
Fliss H, Weissbach H, Brot N: Oxidation of methionine residues in proteins of activated human neutrophils. Proc Natl Acad Sci USA 80:7160–7164, 1983
Gross E: The cyanogen bromide reaction. In: CW Hirs (ed.) Methods in Enzymology, vol 11. Academic Press, New York, 1967, pp 238–255
Guarnieri C, Flamigni F, Caldarera CM: Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J Mol Cell Cardiol 12:797–808, 1980
Sedlak J, Lindsay RH: Estimation of total, protein-bound, and non-protein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25:192–205, 1968
Ellman GL: Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77, 1959
Hohl CM, Altschuld RA, Brierey GP: Effects of calcium on the permeability of isolated adult rat heart cells to sodium. Arch Biochem Biophys 221:197–205, 1983
Farmer BB, Mancina M, Williams ES, Watanabe AM: Isolation of calcium tolerant myocytes from adult rat hearts: review of the literature and description of a method. Life Sci 33:1–18, 1983
Grochowski EC, Ganote CE, Hill ML, Jennings RB: Experimental myocardial ischemic injury. I. A comparison of Stadie-Riggs and free-hand techniques on tissue ultrastructure, water and electrolytes during in vitro incubation. J Mol Cell Cardiol 8:173–187, 1976
Brestel EP: Co-oxidation of luminol by hypochlorite and hydrogen peroxide: implications for neutrophil chemiluminescence. Biochem Biophys Res Commun 126:482–488, 1985
Parish CR, Stanley P: Chemical and biological properties of bacterial flagellin following iodination and oxidation by chloramineT. Immunohistochemistry 9:853–872, 1972
Kikuchi Y, Yamiya N, Nozawa T, Hatano M: Non-destructive detection of methionine sulfoxide in the resilium of a surf clam by solid state 13C-NMR spectroscopy. Eur J Biochem 125:575–577, 1982
Sanchez J, Nikolan BJ, Stumpf PK: Reduction of N-acetyl methionine sulfoxide in plants. Plant Physiol 73:619–623, 1983
Halliwell B, Gutteridge JMC: Free radicals in biology and medicine. Clarendon Press, Oxford, 1985
Engler, Dahlgren MD, Peterson MA, Dobbs A, Schmid-Schönbein GW: Accumulation of polymorphonuclear leukocytes during 3 h experimental myocardial ischemia. Am J Physiol 251:1193–11100, 1986
Roberts ChS, Maclean D, Maroko P, Kloner RA: Relation of early mononuclear and polymorphonuclear cell infiltration to late scar thickness after experimentally induced myocardial infarction in the rat. Basic Res Cardiol 80:202–209, 1985
Engler R, Covell JW: Granulocytes cause reperfusion ventricular dysfunction after 15-minute ischemia in the dog. Cite Res 61:20–28, 1987
Brigham KL: Role of free radicals in lung injury. Chest 89:859–863, 1986
Ward PA, Johnson KJ, Till GO: Oxygen radicals, neutrophils, and acute tissue injury. In: AE Taylor, S Matalon and P Ward (eds) Physiology of Oxygen Radicals. American Physiological Society, Bethesda, 1986, pp 145–150
Stroncek DF, Vercellotti GM, Huh PW, Jacob HS: Neutrophil oxidants inactivate alpha-l-protease inhibitor and promote PMN-mediated detachment of cultured endothelium: protection by free methionine. Arteriosclerosis 6:332–340, 1986
Brittigan BE, Cohen MS, Rosen GM: Detection of the production of oxygen-centered free radicals by human neutrophils using spin trapping. J Leuk Biol 41:349–362, 1987
Kanofsky JR: Catalysis of singlet oxygen production in the reaction of hydrogen peroxide and hypochlorous acid by 1,4-diazabicyclo [2.2.2] octane (DABCO). Biochem Biophys Res Commun 134:777–782, 1986
Ushijima Y, Nakano M: No or little production of singlet molecular oxygen in HOCI or HOCl/H2O2: a model system for myeloperoxidase/H2O2/Cl−. Biochem Biophys Res Commun 93:1232–1237, 1980
Albrich J, McCarthy CA, Hurst JK: Biological reactivity by hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci USA 78:210–214, 1981
Clark RA: Oxidative inactivation of pneumolysin by the myeloperoxidase system and stimulated human neutrophils. J Immunol 136:4617–4622, 1986
Ferrante A, Hill NL, Abell TJ, Pruul H: Role of myeloperoxidase in the killing of Naegleriafowleri by lymphokine-altered human neutrophils. Infect Immun 55:1047–1050, 1987
Johnson RJ, Couser WG, Chi EY, Adler S, Klebanoff SJ: New mechanism for glomerular injury: myeloperoxidasehydrogen peroxide-halide system. J Clin Invest 79:1379–1387, 1987
Vissers MCM, Day WA, Winterbourn CC: Neutrophils adherent to a nonphagocytosable surface (glomerular basement membrane) produce oxidants only at the site of attachement. Blood 66:161–166, 1985
Peppin GJ, Weiss SJ: Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc Natl Acad Sci USA 83:4322–4326, 1986
Severns C, Collins-Lech C, Sohule PG: Effect of temperature on production of hypochorous acid by stimulated human neutrophils. J Lab Clin Med 107:29–35, 1986
Morris JC: The acid ionization constant of HOCI from 5 to 35°. J Phys Chem 70:3798–3805, 1966
Test ST, Lampert MB, Ossanna PJ, Thoene JG, Weiss SJ: Generation of nitrogen-chlorine oxidants by human phagocytes. J Clin Invest 74:1341–1349, 1984
Thomas EL, Grisham MB, Jefferson MM: Myeloperoxidase-dependent effect of amines on functions of isolated neutrophils. J Clin Invest 72:441–454, 1983
Thomas EL, Jefferson MM, Grisham MB: Myeloperoxidasecatalyzed incorporation of amines into proteins: role of hypochlorous acid and dichloramines. Biochem 21:6299–6308, 1982
Thomas EL: Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia Coli. Infect Immun 23:522–531, 1979
Winterbourn CC: Comparative reactivitics of various biological compounds with myeloperoxidase-hydrogen peroxidechloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta 840:204–210, 1985
Coble B-I, Dahlgren C, Hed J, Stendahl O: Myeloperoxidase reduces the opsonizing activity of immunoglobulin G and complement component C3b. Biochim Biophys Acta 802:501–505, 1984
Ahmed H, Aull JL, Williams DE, Worley SD: Inactivation of thymidylate synthase by chlorine disinfectants. Int J Biochem 18:245–250, 1986
Trimm JL, Salama G, Abramson JJ: Sulfhydryl oxidation induces rapid calcium release from sarcoplasmic reticulum vesicles. J Biol Chem 261:16092–16098, 1986
Tsan M-F, Chen JW: Oxidation of methionine by human polymorphonuclear leukocytes. J Clin Invest 65:1041–1050, 1980
Ossanna PJ, Test SJ, Matheson NR, Regiani S, Weiss SJ: Oxidative regulation of neutrophil elastase-alpha-l-proteinase inhibitor interactions. J Clin Invest 77:1939–1951, 1986
Yamasaki RB, Osuga DT, Feeney RE: Periodate oxidation of methionine in proteins. Anal Biochem 126:183–189, 1982
Penner MH, Yamasaki RB, Osuga DT, Babin DR, Meares CF, Feeney RE: Comparative oxidations of tyrosines and methionines in transferrins: human serum trasferrin, human lactatransferrin, and chicken ovotransferrin. Arch Biochem Biophys 225:740–747, 1983
Brot N, Weissbach H: Biochemistry and physiological role of methionine sulfoxide residues in proteins. Arch Biochem Biophys 223:271–281, 1983
Travis J, Beatty K, Matheson N: Oxidation of alpha-lproteinase inhibitor: significance for pathology. In: WH Horl and A Heidland (eds) Advances in Experimental and Medical Biology, vol 167. Plenum Press, New York, 1984, pp 89–96
Carp H, Janoff A: Modulation of inflammatory cell protease-tissue antiprotease interactions at sites of inflammation by leukocyte-derived oxidants. In: G Weissman (ed.) Advances in Inflammation Research, vol 5. Raven Press, New York, 1983, pp 173–201
Carrell RW: α-l-antitrypsin: molecular pathology, leukocytes, and tissue damage. J Clin Invest 78:1427–1431, 1986
Matheson NR, Travis J: Differential effects of oxidizing agents on human plasma α-l-proteinase inhibitor and human neutrophil myeloperoxidase. Biochem 24:1941–1945, 1985
Wasil M, Halliwell B, Hutchinson DCS, Baum H: The antioxidant action of human extracellular fluids: effect of human serum and its protein components on the inactivation of α-1antiproteinase by hypochlorous acid and by hydrogen peroxide. Biochem J 243:219–223, 1987
Lawrence DA, Loskutoff DJ: Inactivation of plasminogen activator inhibitor by oxidants. Biochem 25:6351–6355, 1986
Winterbourn CC, Stern A: Human red cells scavenge extracellular hydrogen peroxide and inhibit formation of hypochlorous acid and hydroxyl radical. J Clin Invest 80:1486–1491, 1987
Oliver CN: Inactivation of enzymes and oxidative modification of proteins by stimulated neutrophils. Arch Biochem Biophys 253:62–72, 1987
Hubbard RC, Ogushi F, Fells GA, Cantin AM, Jallat S, Courtney M, Crystal RG: Oxidants spontaneously released by alveolar macrophages of cigarette smokers can inactivate the active site of α-l-antitrypsin, rendering it ineffective as an inhibitor of neutrophil elastase. J Clin Invest 80:1289–1295, 1987
Levine RL: Covalent modification of proteins by mixed function oxidation. In: S Shaltiel and PB Chock (eds) Current Topics in Cellular Regulation, vol 27. Academic Press, New York, 1985, pp 305–316
Vissers MCM, Winterbourn CC: The effect of oxidants on neutrophil-mediated degradation of glomerular basement membrane collagen. Biochim Biophys Acta 889:277–286, 1986
Dice JF: Molecular determinants of protein half-lives in cells. FASEB J 1:349–357, 1987
Hiss H, Masika M, Eley DW, Korecky B: Oxygen radical mediated protein oxidation in the heart. In: PK Singal (ed.) Free Radicals in the Pathophysiology of Heart Disease. Kluwer Academic Publishers, Boston, 1988, pp 71–90
Ferrari R, Ceconi C, Curello S, Guarnieri C, Caldarera CM, Albertini A, Visioli O: Oxygen-mediated myocardial damage during ischaemia and reperfusion: role of the cellular defences against oxygen toxicity. J Mol Cell Cardiol 17:937–945, 1985
Ward PA: Host-defense mechanisms responsible for lung injury. J Allergy Clin Immunol 78:373–378, 1986
80.Fridovich I, Freeman B: Antioxidant defenses in the lung. In: RM Berne (ed) Annual Review of Physiology, vol 48. Annual Review Inc, Palo Alto, 1986, pp 693–702
Reed DJ, Fariss MW: Glutathione depletion and susceptibility. Pharmacol Rev 36:255–33S, 1984
Ziegler DM: Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. In: CE Richardson, PD Boyer, IB David and A Meister (eds) Annual Review of Biochemistry, vol 54. Annual Reviews Inc, Palo Alto, 1985, pp 305–329
Jenkinson SG, Spence TH, Lawrence RA, Hill KE, Duncan CA, Johnson KH: Rat lung glutathione release: response to oxidative stress and selenium deficiency. J Appl Physiol 62:55–60, 1987
Bryan CL, Jenkinson SG: Species variation in lung antioxidant enzyme activities. J Appl Physiol 63:597–602, 1987
Suttorp N, Toepfer W, Roka L: Antioxidant defense mechanisms of endothelial cells: glutathione redox cycle versus catalase. Am J Physiol 251:C671-C680, 1986
Schraufstatter IU, Revak SD, Cochrane CG: Proteases and oxidants in experimental pulmonary inflammatory injury. J Clin Invest 73:1175–1184, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fliss, H. Oxidation of proteins in rat heart and lungs by polymorphonuclear leukocyte oxidants. Mol Cell Biochem 84, 177–188 (1988). https://doi.org/10.1007/BF00421053
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00421053