Abstract
Background: Multiple pharmacologic treatments have been studied for patients with acute respiratory distress syndrome (ARDS) and acute lung injury (ALI). Our objective was to systematically evaluate this literature to determine the effects of these interventions on important clinical outcomes.
Methods: We searched OVID versions of CENTRAL (The Cochrane Library Issue 3, 2003), MEDLINE (1966-week 2, January 2004), EMBASE (1980-week 4, 2004), CINAHL (1982-week 2, January 2004), and HEALTHSTAR (1995-December 2003); proceedings from four conferences (1994–2003); and bibliographies of review articles and included studies. We included randomized controlled trials (RCTs) of pharmacologic treatments compared with no therapy or placebo for established ARDS and ALI in adults admitted to an intensive care unit, with measurement of early mortality, late mortality, duration of ventilation, ventilator-free days, non-pulmonary organ dysfunction, or adverse events. We excluded trials in other populations incorporating subgroup analyses of patients with ARDS and ALI and studies of nitric oxide, partial liquid ventilation, and fluid and nutritional interventions. Two reviewers independently screened studies and abstracted data from studies included in the analysis. Data were pooled using random effects models where appropriate.
Results: We retrieved 75 potentially relevant articles and abstracts, of which 33 trials randomizing 3272 patients met our selection criteria. Meta-analysis showed no effect on early mortality for alprostadil ([prostaglandin E1] seven studies; 693 patients; relative risk [RR] 0.95; 95% confidence interval [CI], 0.77, 1.17), acetylcysteine (five studies; 235 patients; RR 0.89; 95% CI, 0.65, 1.21), early high-dose corticosteroids (two studies; 180 patients; RR 1.12; 95% CI, 0.72, 1.74), or surfactant therapy (nine studies; 1418 patients; RR 0.93; 95% CI, 0.77, 1.12). Most trials of alprostadil, early high-dose corticosteroids, and surfactant therapy showed more adverse events in the active therapy arm. Single small RCTs demonstrated lower hospital mortality (24 patients, RR 0.20; 95% CI, 0.05, 0.81) with corticosteroids for late phase ARDS and lower 1-month mortality (30 patients, RR 0.67; 95% CI, 0.47, 0.95) with pentoxifylline for patients with metastatic cancer and ARDS. Individual trials of nine additional interventions failed to show beneficial effects on prespecified outcomes.
Conclusions: Effective pharmacotherapy for ARDS is extremely limited. Corticosteroids for late phase ARDS and pentoxifylline for patients with metastatic cancer and ARDS reduced mortality in single small studies. However, further research is required to investigate their potential benefit in the treatment of ALI/ARDS.
Similar content being viewed by others
Background
The acute respiratory distress syndrome (ARDS), first described in 1967,[1]is characterized by diffuse inflammation of the alveolar-capillary membrane in response to various pulmonary and extrapulmonary insults.[2]These insults cause pulmonary injury by direct (e.g. gastric aspiration, pneumonia, inhalational injury, pulmonary contusion) or indirect (e.g. sepsis, trauma, pancreatitis, multiple transfusions of blood products) mechanisms. An American-European Consensus Conference[3]formulated a widely cited definition of ARDS as follows: the acute onset of (i) hypoxemia, with a ratio of the partial pressure of arterial oxygen (PaO2) to the inspired fraction of oxygen (FiO2) of 200mm Hg or less; (ii) bilateral infiltrates on a frontal chest radiograph; and (iii) no clinical evidence of left atrial hypertension or a pulmonary artery occlusion pressure of 18mm Hg or less. Acute lung injury (ALI) includes a milder form of lung injury, with a PaO2/FiO2 ratio of ≤300mm Hg. Almost all patients with ARDS and most with ALI require mechanical ventilation to survive. The mortality of ARDS is high, estimated to be between 34% and 60%.[2]In addition, survivors have a prolonged stay in the intensive care unit (ICU) and demonstrate significant functional limitations, primarily fatigue and muscle weakness, that reduce quality of life and persist for at least 1 year after hospital discharge.[4]
Research on therapy for ARDS has focused on a variety of mechanical ventilation strategies and pharmacologic treatments. Animal and clinical studies, including randomized controlled trials, have demonstrated the role of mechanical ventilation in perpetuating lung injury,[5]leading to macroscopic damage (for example, pneumothorax), diffuse ventilator-induced lung injury, and systemic harm in the form of multiple organ system failure.[6,7]Several randomized trials have compared traditional mechanical ventilation to lung protective ventilation strategies (pressure and volume limited) strategies.[8–12]The largest and most recent study,[8]conducted by the ARDS Network, was stopped after a planned interim analysis showed a clinically and statistically significant reduction in hospital mortality from 39.8% to 31.0% (p = 0.007) using low tidal volume ventilation compared with traditional care. The results of this trial have convinced many clinicians and investigators that lung protective ventilation using tidal volume limitation is the most important therapeutic intervention available for patients with ARDS and ALI. More recently, a meta-analysis[13]of five randomized controlled trials (RCTs) found that lung protective ventilation decreased 28-day mortality in patients with ARDS and ALI.
The pathogenesis of ARDS (extensively reviewed elsewhere)[2,14]provides multiple potential targets for pharmacologic interventions. Regardless of the inciting cause of lung injury, the alveolar-capillary membrane is damaged with leakage of protein-rich edema fluid into alveoli. Alveolar epithelial damage involves the basement membrane and types I and II cells. Injury to type II alveolar epithelial cells leads to reduced levels and functionality of surfactant, causing increased surface tension, atelectasis, and decreased lung compliance. Endothelial damage is associated with numerous inflammatory events. These include neutrophil recruitment, sequestration and activation; formation of oxygen radicals; activation of the coagulation system, leading to microvascular thrombosis with platelet-fibrin thrombi; and recruitment of mesenchymal cells with the production of procollagen. Within the alveolar space, the balance between pro-inflammatory (e.g. tumor necrosis factor [TNF]-α and interleukins [IL]-1, -6, and -8) and anti-inflammatory mediators (e.g. IL-1 receptor antagonist and soluble TNF receptor) favors ongoing inflammation. In summary, the initial lung injury is followed by repair, remodeling, and fibrosing alveolitis.
The diversity of approaches to pharmacologic therapy for ARDS and ALI reflects the complex pathophysiology. Therapies evaluated in randomized trials in humans include corticosteroids, other anti-inflammatory agents, immunomodulating agents, pulmonary vasodilators, antioxidants, and surfactants. The evidence for inhaled nitric oxide (NO), a selective pulmonary vasodilator, was appraised in a recent systematic review,[15]which included five (RCTs) of inhaled NO for acute hypoxemic respiratory failure (including ARDS, ALI, and other diagnoses) in adults and children. The authors concluded that inhaled NO improved oxygenation for up to 72 hours without evidence of effect on the duration of mechanical ventilation or mortality (relative risk [RR] 0.98; 95% confidence interval [CI] 0.66, 1.44). A multicenter RCT published after the systematic review confirmed these clinical findings.[16]
The objective of our systematic review was to evaluate the effects of pharmacologic treatments on important clinical outcomes in patients with established ARDS and ALI. A version of this review is published and will be updated in the Cochrane Database of Systematic Reviews.[17]
Methods
Study Eligibility
We selected studies using the following inclusion criteria: RCT design; adult patients admitted to an ICU with established ARDS or ALI; any pharmacologic treatment compared with no therapy or placebo; measurement of early all-cause mortality (at or before 3 months after randomization), late all-cause mortality (beyond 3 months after randomization), duration of ventilation, number of ventilator-free days to day 28,[18]incidence of non-pulmonary organ dysfunction, or adverse events. The primary outcome was early mortality. We accepted the definitions of adult, ARDS, and ALI used by the primary study authors. Unless otherwise noted, we defined adverse events as those leading to discontinuation of the study medication. In studies without a placebo control, they were defined as ‘serious adverse events’ using authors’ definitions.
We excluded any pharmacologic therapy administered as prophylaxis for ARDS or ALI and studies using nitric oxide, partial liquid ventilation (because this intervention uses a pharmacologic therapy as part of a strategy of mechanical ventilation), fluid management and nutritional therapies. We also excluded subgroups of patients with ARDS or ALI reported in RCTs of interventions for other populations, because of the methodologic limitations of subgroup analyses.[19]
Search Strategy
With the assistance of a professional librarian, we searched OVID versions of CENTRAL (The Cochrane Library Issue 3, 2003), MEDLINE (1966 - week 2, January 2004), EMBASE (1980 - week 4, 2004), CINAHL (1982 - week 2, January 2004), and HEALTHSTAR (1995 - December 2003). We used the MeSH term ‘adult respiratory distress syndrome’ and the text words ‘acute lung injury, shock lung, ARDS’, and (‘acute’ or ‘adult’) ‘respiratory distress’, without applying language restrictions. We used a highly sensitive strategy to retrieve RCTs from MEDLINE[20]and modified it for other databases. Full details of the search strategies are available from the authors. We searched conference proceedings (1994-2003) published in American Journal of Respiratory and Critical Care Medicine, Chest, Critical Care Medicine and Intensive Care Medicine and screened the bibliographies of all retrieved studies and recent review papers[21–38]for additional references.
Data Extraction
Two of the authors independently screened articles and abstracts for inclusion and retrieved all potentially relevant studies. For each study we independently extracted data on population, intervention, outcomes and study methods. We considered the following methodologic features: (i) concealment of allocation; (ii) baseline similarity of treatment and control groups (with respect to age, severity of illness, non-pulmonary organ failures, presence of sepsis, and duration of hospitalization, ICU stay, or mechanical ventilation); (iii) level of blinding (caregivers, data collectors, outcomes assessors); (iv) placebo characteristics that potentially enhanced blinding; (v) cointerventions (standardization or documentation of positive end-expiratory pressure, a lung protective ventilation strategy, weaning protocols, corticosteroids, or other cointerventions); (vi) intention-to-treat analysis (patients analyzed by assigned group and not withdrawn post randomization); and (vii) completeness of follow-up for the outcome of early mortality. We contacted trial authors when we required clarification of the primary outcomes data; four authors[39–42]provided additional information. Disagreements between reviewers were resolved by consensus and in consultation with the third author of this manuscript. We did not systematically evaluate the mechanistic quality of the pharmacologic therapies studied (e.g. whether adequate amounts of the therapeutic agent were delivered to the presumed target for sufficient time to achieve the desired biologic response).
Data Analysis
For each pharmacologic treatment, we pooled the results of studies where permitted by the available data. For studies reporting more than one treatment arm differing in medication dose, we combined data from all doses to determine an overall outcome measure for the treatment arm. We considered ICU and hospital mortality to be early mortality. We used Review Manager software, version 4.2 (Cochrane Collaboration, Oxford, UK) to aggregate data using a random-effects model, calculating an overall RR and a 95% CI for pooled categorical data. We tested the significance of the overall treatment effect using the method of DerSimonian and Laird.[43]For each pooled comparison, we used the Q statistic to test for homogeneity.[44]We considered p = 0.05 to be statistically significant for the test of overall treatment effect and p = 0.10 to be statistically significant for the test of homogeneity. No subgroup analyses or exploratory analyses to explain heterogeneity were planned. We anticipated variability in the reporting of adverse events and therefore did not pool these events across studies.
When pooled analyses included at least five studies, we constructed funnel plots of study precision versus treatment effect, in order to explore the presence of bias arising from the non-publication of small non-beneficial trials.
Results
Overall Description of Included Studies
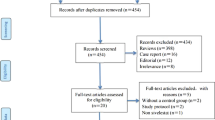
Searches of electronic databases yielded 5705 citations. From these citations, conference abstracts, and bibliographies of retrieved studies, we identified 75 potentially relevant publications. We excluded 42 articles and abstracts for the following reasons: non-randomized;[45–51]cost-effectiveness data from a previously published RCT;[52]no placebo or standard care control group;[53]alternative diagnosis in the study population (at risk for rather than established ARDS or ALI,[54–65]sepsis,[66–70]ventilated patients in surgical ICU,[71]malaria,[72]pancreatitis,[73,74]unilateral lung injury after thoracic aneurysm surgery[75]); outcomes of interest not reported;[76,77]subgroup of patients with ARDS or ALI reported in RCT in another population;[78–83]and duplicate publication.[84–86]
Thirty-three publications describing 33 studies met our inclusion criteria. One paper[39]described two similar but separate studies of surfactant therapy. We counted this paper as two studies. One study of alprostadil (prostaglandin E1) reported mortality data in 147 randomized patients,[87]of whom clinical outcomes in 101 patients had been previously published.[88]We counted these two publications as one study and extracted data on early mortality from the later publication[87]and data on late mortality and adverse events from the earlier publication.[88]One publication[89]reported a trial of patients randomized to receive acetylcysteine, oxothiazolidine carboxylate (procysteine), or placebo. We included the patients randomized to acetylcysteine and placebo in a pooled analysis of the effect of acetylcysteine on early mortality and described the procysteine arm separately. Another study randomized patients to acetylcysteine, acetylcysteine plus rutin (a flavanoid antioxidant), or control.[90]We combined data from both acetycysteine groups in our pooled analysis and did not analyze the effects of rutin, which we classified as a nutritional supplement.
Studies meeting our inclusion criteria randomized 3272 patients and evaluated a wide range of pharmacologic treatments with differing mechanisms of action (table I). They include seven studies of alprostadil (table II),[41,87,88,91–95]five studies of acetylcysteine (table III),[89,90,96–98]three studies of corticosteroids (table IV),[99–101]nine studies of surfactant (table V),[39,40,42,102–106]and single studies (table VI) of dazoxiben,[107]acyclovir,[108]indomethacin,[109]pentoxifylline,[110]neutrophil elastase inhibitor (ICI 200,880),[111]oxothiazolidine carboxylate,[89]interleukin-10,[112]ketoconazole,[113]lisofylline,[114]and granulocyte macrophage colony-stimulating factor (GM-CSF).[115]
Studies of the same pharmacologic therapy differed with respect to patient populations and drug administration as discussed in the following sections.
Studies of Aprostadil
All seven studies of alprostadil [41,87,88,91–95]included patients with predominantly ARDS, although three studies restricted enrollment to those with trauma, surgery, or sepsis as a risk factor.[87,88,92,93]They varied with respect to method of medication administration (continuous infusion[87,88,91–93]versus intermittent boluses[41,94,95]), formulation (liposomal in three trials[41,94,95]) and dose (ranging from 7.2 μg/kg/day[41]to 43.2 μg/kg/day[87,88,91–93]for 7 days).
Studies of Acetylcysteine
The five studies of acetylcysteine [89,90,96–98]included four enrolling primarily patients with ARDS[89,90,96,98]and one that restricted enrollment to those with mild ALI.[97]Two trials used continuous infusions[96,97]and the other three used intermittent intravenous boluses.[89,90,98]The total dose of acetyleysteine varied considerably, from 120 mg/kg[97]to 3510 mg/kg,[96]delivered over 3–10 days. The total dose delivered in one trial[90]was unclear.
Studies of Corticosteroids
The three corticosteroid studies included two trials of high dose, short course (≤48 hours) methylprednisolone early in the course of ALI[99]and ARDS.[100]The duration of ARDS prior to randomization was less than 7 days in both trials. One trial studied a lower dose of methylprednisolone for a longer duration (1 month) in patients with non-resolving ARDS who had been mechanically ventilated for at least 7 days.[101]
Studies of Surfactant
The nine studies of surfactant[39,40,42,102–106]enrolled primarily patients with ARDS. Three restricted eligibility to patients with sepsis-induced ALI or ARDS.[40,102,106]The surfactant preparations were variable: synthetic surfactant with phospholipids,[40,102,106]synthetic surfactant with phospholipids and protein,[39,103,104]bovine surfactant,[42]and porcine surfactant.[105]The method of delivery (continuous aerosolization in early trials[40,102,106]and intratracheal instillation in later trials[39,42,103–105]) and the delivered doses (when measured) were also variable.
Methodologic Assessment of Studies
The methodologic quality of the included studies was variable (table VII). Ten studies[40,87–89,93,100,103,106,113–115]described adequate concealment of allocation. Only four studies did not report at least one prognostically important baseline characteristic,[102,104,105,107]of which three were reported in abstract form.[102,104,105]Of the remaining 29 studies, seven had at least one clinically important imbalance at baseline.[41,93–95,108,112,115]Six studies[39,89,101,106,113]conducted additional analyses adjusting for baseline imbalances. Twenty-six studies reported blinding of at least caregivers or used the term ‘double-blind’. Four of the remaining studies were not placebo-controlled and did not report blinding of any study personnel.[42,103,105,110]One study used a placebo and was described as ‘single-blind’.[107]Two studies explicitly reported no blinding.[90,104]Potential unblinding features associated with study medications used in trials describing caregiver blinding included hypotension with alprostadil[41,87,88,91–95]and dazoxiben,[107]hyperglycemia with corticosteroids,[99–101]and reflux of surfactant into the ventilator tubing.[39,40,102,106]Fourteen studies standardized at least one cointervention or documented its application in the treatment and control groups.[39–42,91,92,95,96,101,103,113–115]The remaining studies did not describe cointerventions. Two ARDS Network trials investigated tidal volume limitation combined with ketoconazole[113]and lisofylline[114]in a factorial design. Of the six other trials[39,41,103,105,115]published after the low tidal volume ventilation study,[8]two[39]documented encouragement of a lung protective ventilation strategy. No study reported imbalances in the application of cointerventions. All studies analyzed patients according to their assigned treatment group; however, 11 reported at least one post-randomization withdrawal[39,42,87–89,95,98,99,108]or did not clearly report this information.[40,107]Twenty-eight studies reported no losses to follow-up; the remaining studies had incomplete follow-up data on fewer than 1% of randomized patients[87,88,95]or did not fully describe the extent of patient follow-up.[40,102,104,114]
Effects of Pharmacotherapies on Clinical Outcomes
We conducted pooled analyses of the effect of alprostadil, acetylcysteine, early corticosteroids and surfactant therapy on early mortality (figure 1, figure 2, figure 3, and figure 4). In all analyses the test for homogeneity was not significant. We were unable to perform pooled analyses for the secondary outcomes (late mortality, duration of mechanical ventilation, ventilator-free days, incidence of non-pulmonary organ dysfunction, adverse events) because of insufficient data and variability in definitions and reporting. Individual trials of these treatments demonstrated no consistent effect on secondary outcomes (table VIII). Effects of additional therapies are summarized separately (table IX).
Effect of alprostadil on mortality in acute respiratory distress syndrome and acute lung injury. The test for homogeneity was nonsignificant (p = 0.27). CI = confidence interval; N = number of patients randomized; n = number of deaths; random = random effects model; RR = relative risk. (See table II for relevant bibliographic references.)
Effect of acetylcysteine on mortality in acute respiratory distress syndrome and acute lung injury. The test for homogeneity was nonsignificant (p = 0.63). CI = confidence interval; N = number of patients randomized; n = number of deaths; random = random effects model; RR = relative risk. (See table III for relevant bibliographic references.)
Effect of early high-dose corticosteroid therapy on mortality in acute respiratory distress syndrome and acute lung injury. The test for homogeneity was nonsignificant (p = 0.16). CI = confidence interval; N = number of patients randomized; n = number of deaths; random = random effects model; RR = relative risk. (See table IV for relevant bibliographic references.)
Effect of surfactant on mortality in acute respiratory distress syndrome and acute lung injury. The test for homogeneity was nonsignificant (p = 0.28). Spragg 2004a (North America) and 2004b (Europe/South Africa) are reported in reference 39. CI = confidence interval; N = number of patients randomized; n = number of deaths; random = random effects model; RR = relative risk. (See table V for relevant bibliographic references.)
Alprostadil
We pooled data from seven trials (figure 1) and found no effect on early mortality (693 patients with outcomes data; RR 0.95; 95% CI, 0.77, 1.17). One study[94]showed a decreased need for ventilation at day 8 but did not use a weaning protocol. Adverse events (figure 5) led to discontinuation of study medication in five trials.[41,91,92,94,95]They occurred more frequently in the alprostadil arm and included cardiopulmonary (hypotension, dysrhythmias, hypoxia) and central nervous system (agitation) events. Data from Bone et al.[88]precluded calculating the total number of patients with adverse events; instead, all patients with hypotension are shown.
Adverse events due to alprostadil, early corticosteroids, and surfactant. Adverse reactions included cardiopulmonary (hypotension, dysrhythmias, hypoxia) and neurological (agitation) events in alprostadil trials; infections in trials of early corticosteroids; and cardiopulmonary events (exhalation valve occlusion, barotrauma, hypoxemia, respiratory arrest, increased peak airway pressure, hypotension, bradycardia) in surfactant trials. CI = confidence interval; N = number of patients randomized; n = number of adverse events; RR = relative risk. (See table II, table IV, and table V for relevant bibliographic references.)
Acetylcysteine
Aggregate analysis of five studies (figure 2) showed no effect on early mortality (235 patients with outcomes data; RR 0.89; 95% CI, 0.65, 1.21). One study [97]showed a decreased need for ventilation on day 3 but did not use a weaning protocol. The five studies reported no adverse events in the placebo group and only one (rash) in the acetylcysteine group,[96]which resolved after acetylcysteine was stopped.
Early High-Dose Corticosteroids
The meta-analysis of two studies (figure 3) showed no effect on early mortality (180 patients with outcomes data; RR 1.12; 95% CI, 0.72, 1.74). The major adverse effect (figure 5) was the development of new infection during the first 7 days. The definition of new infection differed. Bernard et al.[100]excluded positive urine and sputum cultures from catheterized and intubated patients whereas Weigelt et al.[99]did not report excluding them. Both studies demonstrated a statistically significant increase in hyperglycemia with corticosteroid therapy (data not able to be pooled). These adverse effects did not lead to discontinuation of study medications.
Surfactant Therapy
Pooling nine studies (figure 4) demonstrated no effect on early mortality (1418 patients with outcomes data; RR 0.93; 95% CI, 0.77, 1.12). One study assessed the incidence of non-pulmonary organ dysfunction. Gregory et al.[42]followed patients for the development of organ failures (not defined) for 5 days after randomization to surfactant therapy or standard therapy and reported greater cardiovascular failure (not quantified) on day 2 in the high-dose arm compared with controls. Adverse events (figure 5) were not reported to have resulted in discontinuation of the intervention and were primarily cardiopulmonary (for example, exhalation valve occlusion, barotrauma, hypoxemia, respiratory arrest, increased peak airway pressure, hypertension, trachycardia). One trial[103]reported no adverse events leading to discontinuation of therapy.
Evaluation of Publication Bias
Funnel plots of study precision versus treatment effect (not shown) for trials of acetylcysteine, alprostadil, and surfactant therapy suggested the presence of bias arising from non-publication of small non-beneficial trials.
Additional Therapies
One study enrolling 24 patients[101]meeting consensus conference criteria for ARDS[3]showed that administration of corticosteroids for late phase non-resolving ARDS reduced mortality in the ICU (RR 0.05; 95% CI, 0.00, 0.78) and hospital (RR 0.20; 95% CI 0.05, 0.81) and reduced duration of ventilation and organ failure-free days, with no increase in infectious complications. One study of 30 patients[110]with metastatic cancer and ARDS (as defined by the authors) found that pentoxifylline reduced one month mortality (RR 0.67; 95% CI 0.47, 0.95) with no adverse events leading to discontinuation of therapy. There was no evidence of effect of any other intervention (table IX) on prespecified outcomes.
Discussion
The principal finding of our systematic review is that no pharmacotherapy convincingly improves survival in patients with ARDS and ALI. We identified 33 randomized trials of pharmacologic treatments of established ARDS and ALI enrolling 3272 patients, with the first trials being published in 1985.[99,107]The median number of patients randomized per trial was low (45, range 9–725). Our pooled analyses had sample sizes of 180–1418 patients (early high-dose cotricosteroids and surfactant therapy respectively). These analyses showed that alprostadil, acetylcysteine, early administration of high-dose corticosteroids, and surfactant therapy, had no effect on early mortality. With respect to adverse effects, all trials of alprostadil and early high-dose corticosteroids and six out of seven surfactant trials with safety data showed more adverse events with active therapy. However, the potential difficulty of achieving effective blinding for alprostadil and surfactant may have biased adverse event reporting in the active treatment arms. Of the miscellaneous interventions evaluated in single small trials, corticosteroids given late in the course of ARDS decreased ICU and hospital mortality,[101]and pentoxifylline decreased mortality in patients with metastatic cancer and ARDS.[110]
Because the confidence intervals around pooled relative risks for alprostadil, acetylcysteine, early high-dose corticosteroids, and surfactant therapy are wide, we cannot exclude the possibility of a modest benefit or harm to patients. However, in each case the overall best estimate of relative risk suggested no effect. Clinical application of these therapies is therefore not currently justified. Any additional large trials should await pre-clinical and preliminary clinical studies to identify variations of dose, timing of administration, and formulation that may lead to clinical benefit. For example, a recently completed trial of HL 10[122]evaluated the only surfactant preparation shown to be potentially beneficial for ALI/ARDS,[105]a porcine surfactant with a high phospholipid concentration delivered by intratracheal installation to maximize distal airway deposition.
We found that the scientific quality[123]of included trials was variable. Methodologic strengths of a clear majority of studies included caregiver blinding or ‘double-blinding’,[124]analysis of early mortality by strict intention-to-treat criteria (analysis according to assigned group and zero withdrawals), and complete patient follow-up. No study documented differential application of potentially important cointerventions, although the majority provided no information about cointerventions. About one-half of the trials documented similarity between treatment groups for at least one prognostically important baseline characteristic. However, only a minority of studies described adequate allocation concealment. Our assessment of methodologic features was restricted to published descriptions, and we may therefore have underestimated the methodologic strength of some studies, especially those reported in abstract form.[102,104,105,111,112]
Strengths of this systematic review include the use of strategies to minimize bias in the selection and reporting of studies: (i) extensive literature search; (ii) duplicate independent screening of articles and data abstraction; and (iii) explicit criteria for methodologic assessment. [125]We used clinical judgment to decide a priori to combine studies for which a similar direction and magnitude of treatment effect could reasonably be expected. However, aggregated studies differed with respect to eligibility criteria, study drug formulations, methods of administration, and doses. We used random effects models to aggregate data and generate conservative confidence limits for the point estimate of the pooled treatment effect.[126]
Another limitation of this systematic review is the identification of a small number of trials of each pharmacologic therapy, most of which randomized a small number of patients. Funnel plots for trials of acetylcysteine, alprostadil and surfactant therapy suggested the existence of small, unpublished, non-beneficial trials. However, the inclusion of any such trials would not qualitatively change our findings or conclusions.[127]We did not search for RCTs of pharmacologic therapies for other related critically ill populations (such as sepsis) reporting outcomes in subgroups of patients with ALI and ARDS because of limitations in the interpretation of subgroup analyses.
Effective pharmacologic therapy for established ARDS and ALI is therefore extremely limited. We identified two potentially beneficial interventions. First, prolonged corticosteroid administration for late ARDS, which may attenuate the fibroproliferative process, was evaluated in one trial. This study[101]randomized 24 patients with late-phase ARDS and showed an impressive but clinically implausible point estimate of benefit with a wide CI (absolute risk reduction in ICU mortality 0.62; 95% CI, 0.30, 0.95; number needed to treat to prevent one ICU death, 1.6). The authors did not describe concealment of allocation; methodologic strengths include blinding of caregivers, intention-to-treat analysis, complete follow-up, and adjustment for baseline imbalances. However, the major methodologic limitation relates to the data analysis. The investigators calculated a priori that 99 patients would be required to detect an absolute survival improvement of 30% in corticosteroid-treated patients. Enrollment was terminated prematurely using early stopping rules based on sequential clinical trials.[128]However, this approach may inflate the treatment effect in small trials[129]by increasing the probability of confounding by imbalances in baseline prognostic characteristics. Patients in the corticosteroid group had less organ dysfunction and pulmonary morbidity and thus may have had a better prognosis, a bias that may have persisted and confounded the results despite statistical adjustment. In addition, the crossover of four of the eight placebo-assigned patients to corticosteroids (as per protocol) precluded an accurate evaluation of the risk of infection associated with this treatment regimen. Therefore, this trial provides important, albeit preliminary, evidence for the efficacy of corticosteroids for late phase ARDS. The recently completed Late Steroid Rescue Study,[130]which randomized 180 patients, should provide more definitive data.
The second potentially beneficial intervention we identified was administration of pentoxifylline, a phosphodiesterase inhibitor that prevents neutrophil chemotaxis and activation. This therapy was evaluated in a randomized trial enrolling 30 patients with metastatic cancer.[110]Although unclear allocation concealment and lack of blinding threaten the internal validity of this trial, the most important limitation is lack of generalizability. Eighteen of 30 randomized patients had lymphangitic metastases that may have mimicked the characteristic radiographic changes of ARDS, resulting in diagnostic misclassification. The mortality in this trial was higher (25 of 30 patients died at 1 month) than generally observed in patients with ARDS,[2,131]which limits the applicability of the findings to lower-risk patients. Finally, the severity of patients’ lung injury as defined by the widely used American-European Consensus Conference definition[3]was not clear because the authors did not describe the oxygenation criteria used for the diagnosis of ARDS, or whether ventilatory support was required. Further research is required to assess the potential role of pentoxifylline in the treatment of ALI and ARDS in patients without cancer.
There are several potential reasons to explain the large number of non-beneficial trials of pharmacologic therapies for ALI and ARDS. These explanations overlap with insights into the design of RCTs in patients with severe sepsis.[132]First, randomized trials may have been conducted before early investigations had established the optimal dose and duration of the candidate therapy that would achieve adequate tissue levels and biologic response. Second, the definition of the clinical syndrome of ALI and ARDS, although feasible to apply in clinical settings and in research, does not have optimal reliability and validity.[131,133,134]Trials may therefore have enrolled patients unlikely to respond to candidate therapies. However, an inadequate definition of ALI and ARDS cannot be the exclusive explanation for non-beneficial trials given the recent positive RCT of low total volumes in ALI patients.[8]Third, ALI and ARDS are clinically heterogeneous syndromes with various causes, genetic susceptibilities[134]and clinical courses. Trials may thus have included responsive and non-responsive subgroups. Fourth, there are many important non-pulmonary determinants of the outcome for patients with ALI and ARDS. Fifth, investigators may have overestimated the instrinsic therapeutic potential of candidate therapies. The latter three points may have contributed to the design of RCTs that were inadequately powered to reliably detect moderate clinical benefits.
Conclusion
In summary, we found that alprostadil, acetylcysteine, early administration of high-dose corticosteroids, and surfactant therapy had no beneficial effect on survival in critically ill patients with ARDS or ALI. Prolonged corticosteroid administration was effective for late phase ARDS in one small study, and a multicenter RCT[130]of this therapy was recently completed. Pentoxifylline was effective in a small study of highly selected patients with metastatic cancer and ARDS. Given the complex pathophysiology of lung injury, the lack of effective pharmacologic therapies is not surprising. Future treatment may involve a combination of lung-protective ventilation, multi-modality pharmacologic therapy, and earlier identification and better treatment of precipitating factors.
References
Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967 Aug 12; 2: 319–23
Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000 May 4; 342: 1334–49
Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med 1994; 20: 225–32
Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003 Feb 20; 348: 683–93
Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol 2000 Oct; 89: 1645–55
Murphy DB, Cregg N, Tremblay L, et al. Adverse ventilatory strategy causes pulmonary-to-systemic translocation of endotoxin. Am J Respir Crit Care Med 2000 Jul; 162: 27–33
Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999 Jul 7; 282: 54–61
The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000 May 4; 342: 1301–8
Stewart TE, Meade MO, Cook DJ, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome: Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med 1998 Feb 5; 338: 355–61
Brower RG, Shanholtz CB, Fessier HE, et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med 1999 Aug; 27: 1492–8
Brochard L, Roudot-Thoraval F, Roupie E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome: the Multicenter Trial Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med 1998 Dec; 158: 1831–8
Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998 Feb 5; 338: 347–54
Petrucci N, Iacovelli W. Ventilation with lower tidal volumes versus traditional tidal volumes in adults for acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; (2): CD003844
Luce JM. Acute lung injury and the acute respiratory distress syndrome. Crit Care Med 1998 Feb; 26: 369–76
Sokol J, Jacobs SE, Bohn D. Inhaled nitric oxide for acute hypoxemic respiratory failure in children and adults. Cochrane Database Syst Rev 2003; (1): CD002787
Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA 2004 Apr 7; 291: 1603–9
Adhikari N, Burns KEA, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; (4): CD004477
Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002 Aug; 30: 1772–7
Oxman AD, Guyatt GH. A consumer’s guide to subgroup analyses. Ann Intern Med 1992 Jan 1; 116: 78–84
Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol 2002 Feb; 31: 150–3
Bernard GR. Potential of N-acetylcysteine as treatment for the adult respiratory distress syndrome. Eur Respir J Suppl 1990 Oct; 11: 496s–8s
Bernard GR. N-acetylcysteine in experimental and clinical acute lung injury. Am J Med 1991 Sep 30; 91: 54S–9S
Conner BD, Bernard GR. Acute respiratory distress syndrome: potential pharmacologic interventions. Clin Chest Med 2000 Sep; 21: 563–87
McIntyre Jr RC, Pulido EJ, Bensard DD, et al. Thirty years of clinical trials in acute respiratory distress syndrome. Crit Care Med 2000 Sep; 28: 3314–31
Que LG, Huang Y-C. Pharmacologic adjuncts during mechanical ventilation. Semin Respir Crit Care Med 2000; 21: 223–32
Spragg RG. Surfactant replacement therapy. Clin Chest Med 2000 Sep; 21: 531–41
Brower RG, Ware LB, Berthiaume Y, et al. Treatment of ARDS. Chest 2001 Oct; 120: 1347–67
Anzueto A. Surfactant supplementation in the lung. Respir Care Clin N Am 2002 Jun;8: 211–36
Chadda K, Annane D. The use of corticosteroids in severe sepsis and acute respiratory distress syndrome. Ann Med 2002; 34: 582–9
Cranshaw J, Griffiths MJ, Evans TW. The pulmonary physician in critical care part 9: non-ventilatory strategies in ARDS. Thorax 2002 Sep; 57: 823–9
Dos Santos CC, Chant C, Slutsky AS. Pharmacotherapy of acute respiratory distress syndrome. Expert Opin Pharmacother 2002; 3: 875–88
Spragg RG. The future of surfactant therapy for patients with acute lung injury: new requirements and new surfactants. Biol Neonate 2002; 81: 20–4
Tasaka S, Hasegawa N, Ishizaka A. Pharmacology of acute lung injury. Pulm Pharmacol Ther 2002; 15: 83–95
Laterre PF, Wittebole X, Dhainaut JF. Anticoagulant therapy in acute lung injury. Crit Care Med 2003 Apr; 31: S329–36
Lewis JF, Brackenbury A. Role of exogenous surfactant in acute lung injury. Crit Care Med 2003 Apr; 31: S324–8
Lovat R, Preiser J-C. Antioxidant therapy in intensive care. Curr Opin Crit Care 2003; 9: 266–70
Thompson BT. Glucocorticoids and acute lung injury. Crit Care Med 2003 Apr; 31: S253–7
Wiedemann HP, Arroliga AC, Komara Jr JJ. Emerging systemic pharmocologic approaches in acute respiratory distress syndrome. Respir Care Clin N Am 2003; 9: 419–35
Spragg RG, Lewis JF, Walmrath H-D, et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med 2004 Aug 26; 351(9): 884–92
Weg JG, Balk RA, Tharratt RS, et al. Safety and potential efficacy of an aerosolized surfactant in human sepsis-induced adult respiratory distress syndrome. JAMA 1994 Nov 9; 272: 1433–8
Vincent JL, Brase R, Santman F, et al. A multi-centre, double-blind, placebo-controlled study of liposomal prostaglandin E1 (TLC C-53) in patients with acute respiratory distress syndrome. Intensive Care Med 2001 Oct; 27: 1578–83
Gregory TJ, Steinberg KP, Spragg R, et al. Bovine surfactant therapy for patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1997 Apr; 155: 1309–15
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986 Sep; 7: 177–88
Cochran W. The combination of estimates from different experiments. Biometrics 1954; 10: 101–29
van der Merwe CJ, Louw AF, Welthagen D, et al. Adult respiratory distress syndrome in cases of severe trauma: the prophylactic value of methylprednisolone sodium succinate. S Afr Med J 1985 Feb 23; 67: 279–84
Kawai S, Kawahira M, Miura H, et al. Effect of ulinastatin on septic ARDS. Japanese Pharmacol Ther 1988 Aug; 16: 453–9
Kawai S, Sakayori S, Watanabe H, et al. Usefulness of a protease inhibitor (urinastatin) in ARDS with infectious diseases. Nihon Kyobu Shikkan Gakkai Zasshi 1990 Jun; 28: 843–51
Russell JA, Ronco JJ, Dodek PM. Physiologic effects and side effects of prostaglandin E1 in the adult respiratory distress syndrome. Chest 1990 Mar; 97: 684–92
Radermacher P, Huet Y, Pluskwa F, et al. Comparison of ketanserin and sodium nitroprusside in patients with severe ARDS. Anesthesiology 1988 Jan; 68: 152–7
Radermacher P, Santak B, Falke KJ. Comparison of prostaglandin E1 and nitroglycerin in patients with ARDS. Prog Clin Biol Res 1989; 301: 267–70
Lucas CE, Ledgerwood AM. Pulmonary response of massive steroids in seriously injured patients. Ann Surg 1981 Sep; 194: 256–61
Umberger R, Headley A, Waters T, et al. Cost-effectiveness of methylprednisolone treatment (MPT) in unresolving ARDS (U-ARDS) [abstract]. Am J Respir Crit Care Med 2002; 165: A22
Ogawa M, Tamakuma S, Hirasawa H, et al. Effect of a specific neutrophil elastase inhibitor, ONO-5046 Na on the lung dysfunction associated with SIRS (systemic inflammatory response syndrome) [abstract]. Eur Respir J 1996; 9: 286s
Bone RC, Fisher Jr CJ, Clemmer TP, et al. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest 1987 Dec; 92: 1032–6
Nelson S, Belknap SM, Carlson RW, et al. A randomized controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalized patients with community-acquired pneumonia: CAP Study Group. J Infect Dis 1998 Oct; 178: 1075–80
Nelson S, Heyder AM, Stone J, et al. A randomized controlled trial of filgrastim for the treatment of hospitalized patients with multilobar pneumonia. J Infect Dis 2000; 182: 970–3
Tuxen DV, Cade JF. Effect of aprotinin in adult respiratory distress syndrome. Anaesth Intensive Care 1986 Nov; 14: 390–9
Vincent JL, Brimioulle S, Berre J, et al. Prevention of the adult respiratory distress syndrome with dipyridamole. Crit Care Med 1985 Oct; 13: 783–5
Yu M, Tomasa G. A double-blind, prospective, randomized trial of ketoconazole, a thromboxane synthetase inhibitor, in the prophylaxis of the adult respiratory distress syndrome. Crit Care Med 1993 Nov; 21: 1635–42
Schuster DP, Metzler M, Opal S, et al. Recombinant platelet-activating factor acetylhydrolase to prevent acute respiratory distress syndrome and mortality in severe sepsis: phase IIb, multicenter, randomized, placebo-controlled, clinical trial. Crit Care Med 2003 Jun; 31: 1612–9
Slotman GJ, Burchard KW, D’Arezzo A, et al. Ketoconazole prevents acute respiratory failure in critically ill surgical patients. J Trauma 1988 May; 28: 648–54
Bursten SL, Federighi DA, Parsons P, et al. An increase in serum C18 unsaturated free fatty acids as a predictor of the development of acute respiratory distress syndrome. Crit Care Med 1996 Jul; 24: 1129–36
McMichan JC, Rosengarten DS, McNeur JC, et al. Posttraumatic lung syndrome: definition, diagnosis, and therapy: report of a double blind study. Med Welt 1976 Nov 26; 76: 2331–40
Luce JM, Montgomery AB, Marks JD, et al. Ineffectiveness of high-dose methyl-prednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis 1988 Jul; 138: 62–8
McMichan JC, Rosengarten DS, Philipp E. Prophylaxis of post-traumatic pulmonary insufficiency by protease-inhibitor therapy with aprotinin: a clinical study. Circ Shock 1982 Feb; 9: 107–16
Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock: a prospective, controlled study. N Engl J Med 1984 Nov 1; 311: 1137–43
Fourrier F, Chopin C, Huart JJ, et al. Double-blind, placebo-controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest 1993 Sep; 104: 882–8
Tanaka H, Nishino M, Nakamori Y, et al. Granulocyte colony-stimulating factor (G-CSF) stiffens leukocytes but attenuates inflammatory response without lung injury in septic patients. J Trauma 2001 Dec; 51: 1110–6
Wunderink R, Leeper Jr K, Schein R, et al. Filgrastim in patients with pneumonia and severe sepsis or septic shock. Chest 2001 Feb; 119: 523–9
Bernard GR, Reines HD, Halushka PV, et al. Prostacyclin and thromboxane A2 formation is increased in human sepsis syndrome: effects of cyclooxygenase inhibition. Am Rev Respir Dis 1991 Nov; 144: 1095–101
Konrad F, Schoenberg MH, Wiedmann H, et al. The application of N-acetylcysteine as an antioxidant and mucolytic in mechanical ventilation in intensive care patients: a prospective, randomized, placebo-controlled, double-blind study. Anaesthesist 1995 Sep; 44: 651–8
Looareesuwan S, Wilairatana P, Vannaphan S, et al. Pentoxifylline as an ancillary treatment for severe falciparum malaria in Thailand. Am J Trop Med Hyg 1998 6Mar; 58: 348–53
Paran H, Neufeld D, Mayo A, et al. Preliminary report of a prospective randomized study of octreotide in the treatment of severe acute pancreatitis. J Am Coll Surg 1995 Aug; 181: 121–4
Paran H, Mayo A, Paran D, et al. Octreotide treatment in patients with severe acute pancreatitis. Dig Dis Sci 2000 Nov; 45: 2247–51
Satoh D, Matsukawa S, Saishu T, et al. Effect of surfactant on respiratory failure associated with thoracic aneurysm surgery. Crit Care Med 1998 Oct; 26: 1660–2
Heard SO, Longtine K, Toth I, et al. The influence of liposome-encapsulated prostaglandin E1 on hydrogen peroxide concentrations in the exhaled breath of patients with the acute respiratory distress syndrome. Anesth Analg 1999 Aug; 89: 353–7
Bernard GR, Swindell BB, Meredith MJ, et al. Glutathione (GSH) repletion by N-acetylcysteine (NAC) in patients with the adult respiratory distress syndrome [abstract]. Am Rev Respir Dis 1989; 139: A221
Bone RC, Balk RA, Fein AM, et al. A second large controlled clinical study of E5, a monoclonal antibody to endotoxin: results of a prospective, multicenter, randomized, controlled trial. The E5 Sepsis Study Group. Crit Care Med 1995 Jun; 23: 994–1006
Root RK, Lodato RF, Patrick W, et al. Multicenter, double-blind, placebo-controlled study of the use of filgrastim in patients hospitalized with pneumonia and severe sepsis. Crit Care Med 2003 Feb; 31: 367–73
Marshall J, for the Afelimomab Study Group. Effect of anti-TNF antibody on pulmonary component of MOD score [abstract]. Am J Respir Crit Care Med 2001 May; 163: A820
Bigatello LM, Greene RE, Sprung CL, et al. HA-1A in septic patients with ARDS: results from the pivotal trial. Intensive Care Med 1994 May; 20: 328–34
Fisher Jr CJ, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 1994 Jun 15; 271: 1836–43
Bernard GR, Wheeler AP, Russell JA, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis: the Ibuprofen in Sepsis Study Group. N Engl J Med 1997 Mar 27; 336: 912–8
Shoemaker WC. Effectiveness of prostaglandin E1 in adult respiratory distress syndrome. Prog Clin Biol Res 1987; 236A: 361–8
Holcroft JW, Vassar MJ, Weber CJ. Prostaglandin E1 and survival in patients with the adult respiratory distress syndrome: a prospective trial. Semin Respir Med 1986; 7: 40–7
Wiedemann H, Baughman R, DeBoisblanc B, et al. A multicenter trial in human sepsis-induced ARDS of an aerosolized synthetic surfactant (Exosurf) [abstract]. Am Rev Respir Dis 1992; 145: A184
Slotman GJ, Kerstein MD, Bone RC, et al. The effects of prostaglandin E1 on non-pulmonary organ function during clinical acute respiratory failure: the Prostaglandin E1 Study Group. J Trauma 1992 Apr; 32: 480–8
Bone RC, Slotman G, Maunder R, et al. Randomized double-blind, multicenter study of prostaglandin E1 in patients with the adult respiratory distress syndrome: Prostaglandin E1 Study Group. Chest 1989 Jul; 96: 114–9
Bernard GR, Wheeler AP, Arons MM, et al. A trial of antioxidants N-acetylcysteine and procysteine in ARDS: the Antioxidant in ARDS Study Group. Chest 1997 Jul; 112: 164–72
Ortolani O, Conti A, De Gaudio AR, et al. Protective effects of N-acetylcysteine and rutin on the lipid peroxidation of the lung epithelium during the adult respiratory distress syndrome. Shock 2000 Jan; 13: 14–8
Holcroft JW, Vassar MJ, Weber CJ. Prostaglandin E1 and survival in patients with the adult respiratory distress syndrome: a prospective trial. Ann Surg 1986 Apr; 203: 371–8
Rossignon MD, Khayat D, Royer C, et al. Functional and metabolic activity of polymorphonuclear leukocytes from patients with adult respiratory distress syndrome: results of a randomized double-blind placebo-controlled study on the activity of prostaglandin E1. Anesthesiology 1990 Feb; 72: 276–81
Shoemaker WC, Appel PL. Effects of prostaglandin E1 in adult respiratory distress syndrome. Surgery 1986 Mar; 99: 275–83
Abraham E, Park YC, Covington P, et al. Liposomal prostaglandin E1 in acute respiratory distress syndrome: a placebo-controlled, randomized, double-blind, multicenter clinical trial. Crit Care Med 1996 Jan; 24: 10–5
Abraham E, Baughman R, Fletcher E, et al. Liposomal prostaglandin E1 (TLC C-53) in acute respiratory distress syndrome: a controlled, randomized, double-blind, multicenter clinical trial. TLC C-53 ARDS Study Group. Crit Care Med 1999 Aug; 27: 1478–85
Jepsen S, Herlevsen P, Knudsen P, et al. Antioxidant treatment with N-acetylcysteine during adult respiratory distress syndrome: a prospective, randomized, placebo-controlled study. Crit Care Med 1992 Jul; 20: 918–23
Suter PM, Domenighetti G, Schaller MD, et al. N-acetylcysteine enhances recovery from acute lung injury in man: a randomized, double-blind, placebo-controlled clinical study. Chest 1994 Jan; 105: 190–4
Domenighetti G, Suter PM, Schaller MD, et al. Treatment with N-acetylcysteine during acute respiratory distress syndrome: a randomized, double-blind, placebo-controlled clinical study. J Crit Care 1997 Dec; 12: 177–82
Weigelt JA, Norcross JF, Borman KR, et al. Early steroid therapy for respiratory failure. Arch Surg 1985 May; 120: 536–40
Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med 1987 Dec 17; 317: 1565–70
Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 1998 Jul 8; 280: 159–65
Reines HD, Silverman H, Hurst J, et al. Effects of two concentrations of nebulized surfactant (Exosurf) in sepsis-induced adult respiratory distress syndrome (ARDS) [abstract]. Crit Care Med 1992 Apr; 20: S61
Spragg RG, Lewis JF, Wurst W, et al. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med 2003 Jun 1; 167: 1562–6
Walmrath D, De Vaal JB, Bruining HA, et al. Treatment of ARDS with a recombinant SP-C (rSP-C) based synthetic surfactant [abstract]. Am J Respir Crit Care Med 2000; 161: A379
Kesecioglu J, Schultz MJ, Lundberg D, et al. Treatment of acute lung injury (ALI/ARDS) with surfactant [abstract]. Am J Respir Crit Care Med 2001 May; 163: A819
Anzueto A, Baughman RP, Guntupalli KK, et al. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome: Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. N Engl J Med 1996 May 30; 334: 1417–21
Reines HD, Halushka PV, Olanoff LS, et al. Dazoxiben in human sepsis and adult respiratory distress syndrome. Clin Pharmacol Ther 1985 Apr; 37: 391–5
Tuxen DV, Wilson JW, Cade JF. Prevention of lower respiratory herpes simplex virus infection with acyclovir in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1987 Aug; 136: 402–5
Steinberg SM, Rodriguez JL, Bitzer LG, et al. Indomethacin treatment of human adult respiratory distress syndrome. Circ Shock 1990 Apr; 30: 375–84
Ardizzoia A, Lissoni P, Tancini G, et al. Respiratory distress syndrome in patients with advanced cancer treated with pentoxifylline: a randomized study. Support Care Cancer 1993 Nov; 1: 331–3
Gottlieb JE, Elmer M, Steiner R. Efficacy of intravenous ICI 200,880, a neutrophil elastase inhibitor, in the treatment of adult respiratory distress syndrome (ARDS) [abstract]. Chest 1994; 106: 67S
Bernard GR, Wheeler AP, Naum CC, et al. A placebo-controlled, randomized trial of IL-10 in acute lung injury (ALI) [abstract]. Chest 1999; 116: 206S
The ARDS Network. Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2000 Apr; 283: 1995–2002
The ARDS Clinical Trial Network. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med 2002 Jan; 30: 1–6
Presneill JJ, Harris T, Stewart AG, et al. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med 2002 Jul 15; 166: 138–43
Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome [published erratum appears in Am Rev Respir Dis 1989 Apr; 139 (4): 1065.]. Am Rev Respir Dis 1988 Sep; 138: 720–3
Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: on behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996 Jul; 22: 707–10
Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985 Oct; 13: 818–29
Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991 Dec; 100: 1619–36
Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995 Oct; 23: 1638–52
Le Gall JR, Loirat P, Alperovitch A, et al. A simplified acute physiology score for ICU patients. Crit Care Med 1984 Nov; 12: 975–7
Leo Pharma. HL 10 phase III study [online]. Available from URL: http://www.leopharma.com/w-site/leo/docs.nsf/2addbdd119e92fb08025690a004eb625/797437f3e23f3e95cl256be50024818e?.OpenDocument [Accessed 2004 Apr 20]
Guyatt G, Cook D, Devereaux PJ, et al. Therapy. In: Guyatt G, Rennie D, editors. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago: AMA Press, 2002: 55–80
Devereaux PJ, Manns BJ, Ghali WA, et al. Physician interpretations and textbook definitions of blinding terminology in randomized controlled trials. JAMA 2001 Apr 18; 285: 2000–3
Oxman A, Guyatt G, Cook D, et al. Summarizing the evidence. In: Guyatt G, Rennie D, editors. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago: AMA Press, 2002: 155–73
Montori V, Guyatt G, Oxman A, et al. Summarizing the evidence: fixed-effects and random-effects models. In: Guyatt G, Rennie D, editors. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago: AMA Press, 2002: 539–46
Begg CB. Publication bias. In: Cooper H, Hedges LV, editors. The handbook of research synthesis. New York: Russell Sage Foundation, 1994: 399–409
Whitehead J, Stratton I. Group sequential clinical trials with triangular continuation regions. Biometrics 1983 Mar; 39: 227–36
Marras T, Herridge M, Mehta S. Corticosteroid therapy in acute respiratory distress syndrome. Intensive Care Med 1999 Oct; 25: 1191–3
ARDS Network. Late steroid rescue study [online]. Available from URL: http://www.ardsnet.org/documents/lasrs6200web.pdf [Accessed 2004 Apr 20]
Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med 2003 Apr 1; 31: S276–84
Opal SM. Clinical trial design and outcomes in patients with severe sepsis. Shock 2003 Apr; 20: 295–302
Ferguson ND, Davis A, Chan CK, et al. Development of a modified clinical definition of ARDS using the delphi technique [abstract]. Am J Respir Crit Care Med 2001; 163: A449
Villar J, Flores C, Mendez-Alvarez S. Genetic susceptibility to acute lung injury. Crit Care Med 2003; 31(4 Suppl.): S272–5
Acknowledgment
The authors thank Elizabeth Uleryk for assistance in searching bibliographic databases, Dr Jan Friedrich and Dr Yumiko Imai for assistance in translation, and Dr Deirdre Grady for assistance in searching conference proceedings.
This study received no funding. Dr Meade is a Peter Lougheed Scholar of the Canadian Institutes of Health Research. Dr Burns holds a postdoctoral fellowship from Merck Frosst and the Canadian Lung Association. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adhikari, N., Burns, K.E.A. & Meade, M.O. Pharmacologic Treatments for Acute Respiratory Distress Syndrome and Acute Lung Injury. Treat Respir Med 3, 307–328 (2004). https://doi.org/10.2165/00151829-200403050-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00151829-200403050-00005