Article Text
Abstract
Rationale: Lower airway (LAW) infection with Pseudomonas aeruginosa and Staphylococcus aureus is the leading cause of morbidity in cystic fibrosis (CF). The upper airways (UAW) were shown to be a gateway for acquisition of opportunistic bacteria and to act as a reservoir for them. Therefore, tools for UAW assessment within CF routine care require evaluation.
Objectives: The aims of the study were non-invasive assessment of UAW and LAW microbial colonisation, and genotyping of P aeruginosa and S aureus strains from both segments.
Methods: 182 patients with CF were evaluated (age 0.4–68 years, median 17 years). LAW specimens were preferably sampled as expectorated sputum and UAW specimens by nasal lavage. P aeruginosa and S aureus isolates were typed by informative single nucleotide polymorphisms (SNPs) or by spa typing, respectively.
Results: Of the typable S aureus and P aeruginosa isolates from concomitant UAW- and LAW-positive specimens, 31 of 36 patients were carrying identical S aureus spa types and 23 of 24 patients identical P aeruginosa SNP genotypes in both compartments. Detection of S aureus or P aeruginosa in LAW specimens was associated with a 15- or 88-fold higher likelihood also to identify S aureus or P aeruginosa in a UAW specimen from the same patient.
Conclusions: The presence of identical genotypes in UAW and LAW suggests that the UAW play a role as a reservoir of S aureus and P aeruginosa in CF. Nasal lavage appears to be suitable for non-invasive UAW sampling, but further longitudinal analyses and comparison with invasive methods are required. While UAW bacterial colonisation is typically not assessed in regular CF care, the data challenge the need to discuss diagnostic and therapeutic standards for this airway compartment.
Trial registration number: NCT00266474.
Statistics from Altmetric.com
Cystic fibrosis (CF) is the most frequent life-threatening recessive genetic disorder in Caucasians. It is caused by mutations in the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) gene. Chronic pulmonary infection with opportunistic bacteria is the major cause of morbidity and mortality in CF. Staphylococcus aureus is one of the first pathogens infecting CF airways for extended periods.1 2 Later, up to 80% of adults with CF are chronically colonised with Pseudomonas aeruginosa,3 indicating progression of the pulmonary destruction4 although aggressive antibiotic treatment can improve life expectancy.5 The natural course of the disease can be ameliorated by preventing or postponing chronic P aeruginosa colonisation. P aeruginosa eradication is possible in early colonisation as long as colonies do not evolve to mucoid phenotypes. Therefore, further understanding of pathways leading to P aeruginosa acquisition is a key issue of contemporary CF research.
Similar to the lower airways (LAW), mucociliary clearance of the upper airways (UAW) is impaired by the causative CFTR defect. Therefore, mucus retention, chronic inflammation, and colonisation of the UAW with opportunistic bacteria are typical in CF. Chronic rhinosinusitis and nasal polyps are pathognomonic signs of the disease.6–8 Resulting symptoms are chronic nasal congestion, rhinorrhoea with anterior or postnasal drip, mouth-breathing, facial pain, anosmia and sleep disturbances, which impair overall health. Altogether, 30–67% of the patients are reported to suffer from chronic rhinosinusitis over all age groups.7 8 Morphological abnormalities of the paranasal sinuses are detected by CT in almost 100% of patients,9 although in some cases this is due to hypoplasic frontal sinuses.
Sinonasal involvement in CF has been proposed as the major source for chronic bronchopulmonary infection with opportunistic bacteria.7 S aureus and P aeruginosa are known to colonise the UAW in those with CF10 and therefore this site may function as a gateway and reservoir for subsequent pulmonary infection. If this were generally true in CF, the same bacterial clones should inhabit the UAW and LAW, and the detection of early airway colonisation in UAW and subsequent therapeutic intervention could accordingly improve the success rate of effective eradication.11 However, despite its potential impact on the management of CF, the association between UAW and LAW colonisation has never been systematically investigated. Hence, we set up a prospective clinical study to compare the microbial flora at the same time in upper and lower CF airways and to assess the genetic relatedness between S aureus and P aeruginosa strains inhabiting the UAW and LAW of subjects with CF. Moreover, non-invasive and simple methods for sampling the UAW that are applicable to daily clinical practice were compared in terms of their sensitivity.
MATERIALS AND METHODS
Patients
A total of 187 patients from five German CF outpatient clinics were enrolled in the study between December 2005 and April 2007. Microbiological sampling from both UAW and LAW was performed for 182 patients.
The inclusion criterion was an established diagnosis of CF confirmed by at least three positive sweat tests and/or two disease-causing CFTR mutations. Exclusion criteria were sinonasal surgery ⩽6 months prior to recruitment and systemic antimicrobial P aeruginosa therapy. Treatment with inhalative aminoglycosides and oral macrolides was reported. Patients were excluded from nasal lavage if they suffered from recurrent nasal bleeding, if the tympanum was perforated and/or if cooperation was not possible (age or other reasons).
Informed written consent was obtained from patients and their parental guardians. The study was approved by the local ethics committees.
Rhinoscopy
Nasal examination performed by flexible or rigid endoscopy included assessment of the mucosal status, sizing of nasal polyps, if applicable, and evaluation of secretions, crusts and other pathological alterations.
Nasal lavage
Nasal lavage was performed by inserting 10 ml of sterile isotonic saline into each nostril with a 10 ml syringe with a slightly reclined position of the head during occlusion of the soft palate (fig 1), as performed during standard therapeutic nasal lavage.12 Prior to the collection of nasal lavage, visual aids and verbal instructions were provided. Samples with contamination from the oral cavity were discarded and the procedure was repeated until non-contaminated samples were retrieved.
Method of diagnostic nasal lavage.
In general, patients older than 6 years were able to perform nasal lavage accurately. Alternatively, deep nasal swabs or swabs of nasal secretions sneezed into a handkerchief (blowing samples) were taken. Additionally, sputum samples or deep throat swabs were obtained. Specimens were processed by the local microbiology laboratory according to the German quality assurance guidelines for CF microbiology.13
Molecular typing of P aeruginosa and S aureus
P aeruginosa isolates were typed in 16 SNPs with a custom-made microarray as described previously.14 DNA amplified from the bacterial colony by cycles of multiplex primer extension was hybridised onto the microarray to yield an electronically portable binary multimarker genotype.14 S aureus isolates were analysed by spa typing. Briefly, the variable region of protein A was amplified with the following primers: spa-1113f (5′-TAAAGACGATCCTTCGGTGAGC-3′) and spa-1514r (5′-CAGCAGTAGTGCCGTTTGCTT-3′)15 and sequenced. Spa types were determined with the Ridom StaphType software.16 Numeric spa repeat and type codes were assigned.
Statistics
Data were evaluated with SPSS for Windows Version 11.5.1 (SPSS, Chicago, Illinois, USA) and Microsoft Excel 2003 (Microsoft, Redmond, Washington, USA). We performed explorative analyses of data. The association between detection of bacteria and fungi in the UAW and LAW was estimated by phi-analysis. Agreement or concordance between both compartments (beyond what would be expected by chance) was measured by Cohen’s kappa. p Values of kappa below 0.05 (global alpha) indicate a statistically significant difference from only chance agreement. Bonferroni adjustment was applied to control alpha for multiple comparisons. To describe the relative risk of finding bacteria or fungi in a UAW sample, we used the odds of finding them in LAW-positive and in LAW-negative subjects and calculated odds ratios (ORs) and their 95% CIs.
RESULTS
Demographic data
Baseline data from the 187 patients with CF (81 females/106 males) recruited in the five outpatient clinics are presented in table 1.
The mutation F508del was detected in 163/187 patients (87.7%), with 78 (41.7%) being homozygous for this most frequent CFTR defect in the German CF population. G551D was detected in 20 patients (5.3%), N1303K and G542X in 10 patients each (2.7%), R553X in 9 patients (2.4%), R347P in 8 patients (2.1%) and 3849+10kbC>T in 7 patientss (1.9%). Exogenous pancreatic insufficiency was present in 175/187 patients (93.6%), and 25/187 (13.4%) were diabetic. Altogether, allergic sensitisation was found in 89/145 (61.4%) assessed patients by detection of specific immunoglobulin E (IgE) in serum. Of the 187 patients with CF, 36 (19.3%) presented with allergic rhinitis and 14/187 (7.5%) fulfilled the criteria for allergic bronchopulmonary aspergillosis (ABPA), according to the CF consensus guidelines.19 ENT (Ear, nose and throat) surgery had previously been performed in 79 of 187 recruited patients with CF, corresponding to a total percentage of 42.2%.
Upper and lower airway colonisation
A total of 182 patients were assessed for microbial colonisation in LAW and UAW (see table 2).
Haemophilus influenzae was found in 9.9% (18) of LAW and 6.0% (11) of UAW samples, and in 6 of these, H influenzae was detected in both compartments. Aspergillus fumigatus was recovered in 14.3% (26/182) of LAW cultures, whereas it grew in only one UAW sample that had been retrieved from a patient who simultaneously had a positive LAW culture. Candida albicans was found in 18.1% (33) of the LAW cultures but in none of the UAW samples.
History of chronic colonisation of the airways with P aeruginosa was defined according to Kerem et al20 as having at least 50% of P aeruginosa-positive LAW samples during a period of 12 months. A total of 63 of 182 (34.6%) patients fulfilled the criteria of history of chronic colonisation with P aeruginosa
P aeruginosa could be identified in UAW samples from 29 (46.0%) of the 63 patients with a history of chronic P aeruginosa colonisation of the LAW. At the same time, the bacterium was found in 90.5% (57/63) of the LAW cultures from these patients. The proportion of patients with detection of P aeruginosa in both airway segments increased with age13 (fig 2).
Fraction of patients with detection of Pseudomonas aeruginosa (Pa) in the upper (UAW) and lower airways (LAW) in relation to their age (n = 182).
In one patient with a history of chronic pulmonary colonisation, P aeruginosa could only be detected in the nasal lavage but not in sputum. P aeruginosa was 88-fold more likely to be detectable in a UAW sample from a LAW P aeruginosa-positive subject than from a LAW P aeruginosa-negative subject (95% CI 11.5 to 667.5).
S aureus was recovered in 55 UAW (30%) and 64 (35%) LAW specimens. S aureus isolates of 4/64 LAW specimens were methicillin-resistant S aureus (MRSA). Samples from 41/64 (64%) patients were positive for S aureus in both the UAW and LAW specimens. Samples from 14 patients were only positive for S aureus in the UAW specimen, while samples from 23 patients were only positive in LAW specimens. S aureus colonisation of both airway sites was found in all age groups (fig 3). A statistically significant association for S aureus carriage in both airway sites was found (Φ = 0.568, p<0.001). The OR analysis revealed a LAW S aureus carrier to be 15.4-fold more likely to be S aureus positive in the UAW than a LAW S aureus-negative subject (95% CI 7.1 to 33.4).
Patients with Stapylococcus aureus (Sa) in the upper (UAW) and lower airways (UAW) in relation to age (n = 182).
Patients with a history of chronic P aeruginosa colonisation less frequently carried S aureus in the UAW and LAW. S aureus was found in the UAW of 14.3% (9/63) of the patients with chronic P aeruginosa colonisation of the LAW, but in 38.7% (46/119) of the patients without chronic P aeruginosa colonisation. Accordingly, the OR was 3.8 higher for nasal S aureus detection in P aeruginosa-negative patients (95% CI 1.7 and 8.4).
Genetic relatedness of P aeruginosa and S aureus strains detected in the UAW and LAW
P aeruginosa strains of 29 patients were typed in 16 informative SNPs of the core genome.14 Isolates from both UAW and LAW were available from 24 patients (LAW samples, 21 expectorated sputum and 3 deep throat swabs; UAW, all 24 obtained by nasal lavage). Strain pairs with identical P aeruginosa SNP marker genotype had been retrieved from the UAW and LAW specimens of 23 patients. Distinct P aeruginosa genotypes had been recovered from the UAW and LAW of only one patient (fig 4A). The isolates were classified into 21 different genotypes including eight common genotypes that each make up >1% of the P aeruginosa population.14 Seven genotypes of this study had not been observed before in our collection of >2000 strains that represent 180 independent clones.
Genetic relatedness of (A) Pseudomonas aeruginosa and (B) Staphylococcus aureus isolates from the upper and lower airways of individuals with cystic fibrosis.
S aureus isolates from 36 patients were analysed further. The isolates were cultured from nasal swabs (n = 30) or nasal lavage (n = 14) representative for the UAW, or from deep throat swabs (n = 24) or sputum (n = 12) representative for the LAW. All S aureus isolates were subjected to spa typing15 that allows comparison of the S aureus isolates not only within the same patient but also between different patients and between different centres. In most patients, the isolates from the UAW and LAW were genotypically identical (31/36; 86.1%) (fig 4B). More than one S aureus genotype was retrieved from throat swabs of two patients. The isolates of 36 patients revealed 37 different spa types. Only two of three MRSA couplets from both airway segments were available for typing. They were found to be genotypically identical in the UAW and LAW for each patient.
Assessment of different methods to sample the UAW and LAW
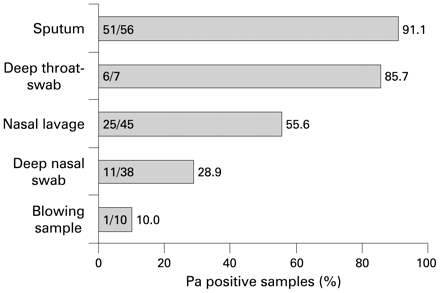
Comparison of different methods to sample material from the UAW of 61 patients revealed remarkable discrepancies in the rate of P aeruginosa-positive cultures from patients with a history of chronic pulmonary P aeruginosa colonisation. P aeruginosa was detected in 56% of nasal lavages (25/45, Φ = 0.648. p<0.001), 29% of deep nasal swabs (11/38, Φ = 0.469, p<0.001) and 10% of blowing samples (1/10, Φ = 0.182, p = 0.667). In the case of the LAW specimens, P aeruginosa was detected in 51 of 56 sputa and 6 of 7 deep throat swabs (fig 5).
Recovery of P aeruginosa (Pa) in specimens taken from the upper (UAW) or lower airways (LAW) of patients with a history of chronic P aeruginosa colonisation of the lower airways (UAW, n = 183; LAW, n = 184).
Of the 53 patients whose UAW were sampled simultaneously with nasal lavage and nasal swab, 7 patients were P aeruginosa positive with both methods, 4 patients P aeruginosa were positive only with lavage, and one patient only with nasal swab.
DISCUSSION
Chronic rhinosinusitis is a hallmark of CF.6–9 The local symptoms frequently impair the quality of life, but—more importantly—chronic rhinosinusitis may remodel the UAW to become a niche for opportunistic pathogens that may descend into the lungs during periods of damage of the oropharyngeal barrier.
This study simultaneously sampled the UAW and LAW of patients with CF with non-invasive methods. The cultured bacteria and fungi were classified into 30 groups (table 2). In 50% of cases, the same microorganism was also recovered from the lungs if it had been cultured from the UAW. A few pathogens were predominantly recovered from only one habitat. Coagulase-negative staphylococci, Corynebacteria and Moraxella spp. were frequently cultured from the UAW, but infrequently from the lungs, in accordance with their preponderance in the nasopharyngeal tract as their natural niche in healthy carriers.21 On the other hand, opportunistic pathogens21 that typically are absent in the commensal flora of healthy individuals were more frequently cultured from CF LAW than from CF UAW. Examples are Candida and Aspergillus, H influenzae and H parainfluenzae, Stenotrophomonas maltophilia and the leading pathogen in CF, P aeruginosa (table 2).
Mucus retention and insufficient aeration of the paranasal sinuses, and nasal polyps and congestion are obligatory symptoms in CF. Hence, one may wonder why the reasonable and fundamental question of whether the UAW are a reservoir and gateway for microbial colonisation in CF has so far been addressed in only a few studies.22–24 The anatomical barriers to retrieve samples are one obvious reason. The direct assessment of the paranasal sinuses requires quite invasive methods such as endoscopy-guided sinus aspiration,25 sinus puncture or intraoperative sampling,22 which are unsuitable for routine clinical management. Thus, this study evaluated an alternative option of non-invasive methods to sample the UAW for bacteriological analysis. The detection of P aeruginosa in UAW specimens retrieved from patients with chronic airway colonisation with P aeruginosa was taken as the standard for comparison. Recovery of P aeruginosa from LAW specimens (sputum or deep throat swab) was about 90%. Nasal lavage turned out to be the method of choice for sampling the UAW, with a recovery rate of close to 60% of P aeruginosa-positive cultures. If subjects were unable to perform the manoeuvre, blowing samples or deep nasal swabs were taken. However, these alternative procedures were not sensitive enough and therefore have to be classified as inappropriate.
The sensitivity of nasal lavage can probably be improved with the subject’s increasing experience in sampling. Although we provided meticulous visual and oral instructions to each study subject, some patients did not adhere to the protocol. Instead of rinsing the full volume of 10 ml of saline, they just applied portions of 2–3 ml with their head in the forward position. Supposedly these individuals predominantly rinsed the nasal vestibulum, but not the nasal conchae with the orifices of the paranasal sinuses. In conclusion, nasal lavage seems to be a promising technique for sampling the UAW in clinical practice. The subject needs to be trained as is necessary for lung function testing. Since both methods require the subject’s cooperation, they are not suitable for young children. However, it is exactly this group of young minors who would benefit most from an early detection of microbial pathogens in the UAW prior to their descent into the LAW. In the case of the major pathogen P aeruginosa, numerous studies have convincingly demonstrated a 80% success rate of eradication of P aeruginosa from the airways5 26 during the stage of early colonisation prior to bacterial adaptation to and diversification in the niche.27 28 Considering the impact of the prevention and therapy of infection, the protocols of UAW sampling for bacteriological analysis, particularly for the risk group of young children, should be further optimised in prospective trials and then introduced into the management of CF.
S aureus and P aeruginosa are the dominant pathogens in CF.1 3 28 Therefore, the major goal of our multicentre study was the analysis of the genetic relatedness of S aureus and P aeruginosa strains that had been retrieved at the same time from the UAW and LAW of a subject with CF. To address this issue, the currently most informative molecular typing methods for S aureus15 and P aeruginosa14 were applied. The outcome was unequivocal; 86% of the S aureus strain pairs and 95% of the P aeruginosa strain pairs were genotypically identical. In other words, the majority of S aureus carriers and almost all P aeruginosa carriers are harbouring the same clones in the upper airways and the lungs. Therefore, the UAW and LAW of a patient with CF are colonised with the same populations of S aureus and P aeruginosa.
This major finding based on the prospective analysis of a CF cohort of all age groups and grades of disease severity is supported by the few published data on preselected patient subgroups.22–24 Walter et al24 typed LAW P aeruginosa isolates from patients prior to and after lung transplantation. Within a few months, the P aeruginosa-free donor lungs became colonised with the P aeruginosa genotype of the explanted lungs. The authors concluded that the patients’ UAW were the reservoir for the colonisation of the lung transplant. During end-stage CF pulmonary disease, the UAW and LAW were colonised with the same P aeruginosa clones. This conclusion was found to be true also for less advanced stages of lung disease. Taylor et al23 identified the same P aeruginosa macrorestriction fragment genotypes in sputa and UAW samples from adults with CF. Recently, Muhlebach et al22 compared the bacteriology of the sinus with that of oropharyngeal swabs and bronchoalveolar lavage fluid (BALF) in children with CF. Samples were taken during operations on subjects requiring sinonasal surgery. If S aureus or P aeruginosa were isolated from both the sinus and the LAW specimen, the concordance of genotypes was 83%. This figure corresponds reasonably well with our data on LAW and UAW strain pairs retrieved by non-invasive procedures.
Considering the possible role of the UAW as a reservoir for lung infections with the major CF pathogens that has been demonstrated by both our study and that of Muhlebach et al, future comparative investigations on the recovery of culturable microbes by invasive and non-invasive sampling of the UAW will be of great interest. If the current protocol of nasal lavage or a modified version would achieve similar detection rates to the direct analysis of sinonasal tissue, nasal lavage could become a routine measure for the bacteriological surveillance in CF and a valid surrogate parameter for antimicrobial intervention trials. Lastly, our results disprove earlier claims29 30 that the sinonasal compartment is not a reservoir for the descending infection to the lung in CF. On the contrary, as several authors had postulated previously,23 31–33 the UAW appear to play a role in the acquisition and persistence of opportunistic bacteria in CF which requires further investigation.34
Acknowledgments
We thank Dr Guenther Frey and Dr Karin Thoss for enrolling patients from their outpatient clinics, and Christiane Ritschel from the Jena University CF study group for the substantial organisational help.
REFERENCES
Footnotes
Competing interests: None.
Funding: The study was supported by funds from Hoffmann-La Roche, Novartis, InfectoPharm, Pari and Gruenenthal. We confirm that the financial support did not influence any aspect of the study and that no other financial resources were provided.
Contributors: JM was principal investigator. LN and MK conducted and supervised the study in participating CF centres. SM and GS were coinvestigators in participating ENT units. JM contributed to the concept and design of the study together with BT, JFB, IS and MS. BT, BCK, LW, NC and WP performed the microbiological analyses including genotyping of P aeruginosa and S aureus strains. BT, BCK, IS, MS and BW contributed to the analysis and interpretation of data.
Patient consent: Obtained.
Ethics approval: The trial was approved by the Jena University Ethics Committee.